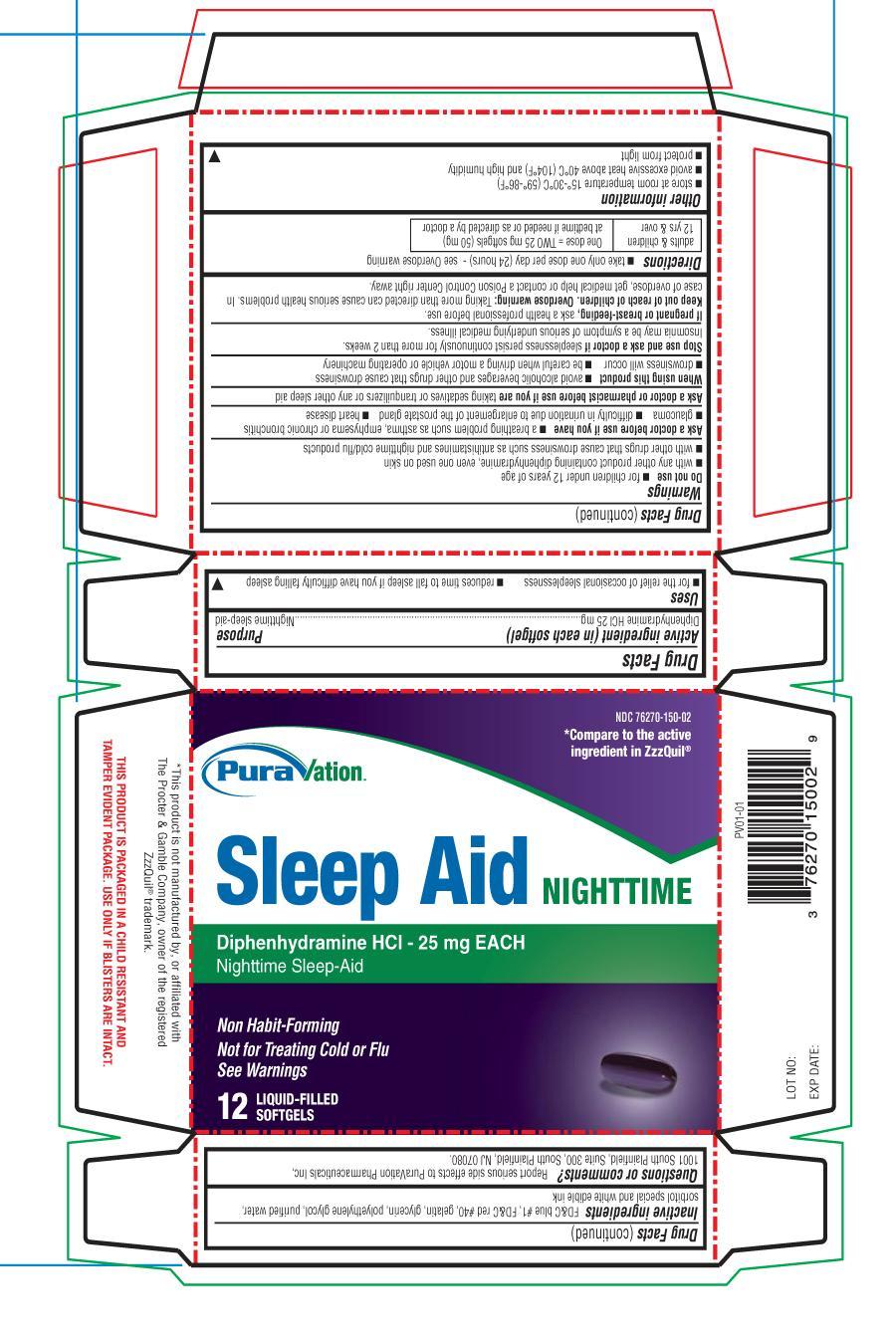
SLEEP AID- diphenhydramine hydrochloride 25 mg capsule, liquid filled
PuraVation Pharmaceuticals Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SLEEP AID (Diphenhydramine 25 mg) capsule, liquid filled
Uses
- for the relief of occasional sleeplessness
- reduces time to fall asleep if you have difficulty falling asleep
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- with other drugs that cause drowsiness such as antihistamines and nighttime cold/flu products
Ask a doctor before use if you have
- a breathing problem such as asthma, emphysema, or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- heart disease
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers or any other sleep aid
When using this product
- avoid alcoholic beverages and other drugs that cause drowsiness
- drowsiness will occur
- be careful when driving a motor vehicle or operating machinery
Directions
- take only one dose per day (24 hours) - see Overdose warning
|
adults & children 12 yrs & over |
One dose = TWO 25 mg softgels (50 mg) at bedtime if needed or as directed by a doctor |
Other information
- store at room temperature 15°-30°C (59°-86°F)
- avoid excessive heat above 40°C (104°F) and high humidity
- protect from light
| SLEEP AID
diphenhydramine hydrochloride 25 mg capsule, liquid filled |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - PuraVation Pharmaceuticals Inc (968385034) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Humanwell PuraCap Pharmaceutical (Wuhan), Ltd. | 421293287 | MANUFACTURE(76270-150) , ANALYSIS(76270-150) | |
Revised: 12/2016
Document Id: 2a505dca-36bb-48f3-8e5f-4d56ec8babc0
Set id: 59e17018-0ae4-4bcf-9d15-3554b6c97069
Version: 5
Effective Time: 20161230
PuraVation Pharmaceuticals Inc