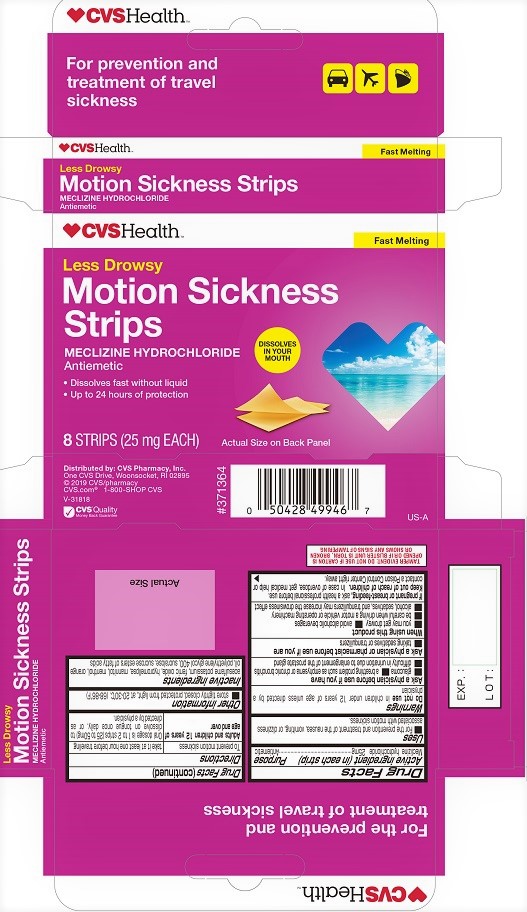
CVS MOTION SICKNESS STRIPS- meclizine hydrochloride film, soluble
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CVS Motion Sickness Strips
Uses
■ For the prevention and treatment of the nausea, vomiting, or dizziness associated with motion sickness.
Warnings
Ask a physician before use if you have
■ glaucoma ■ a breathing problem such as emphysema or chronic bronchitis
■ difficulty in urination due to enlargement of the prostate gland
When using this product
■ you may get drowsy ■ avoid alcoholic beverages
■ be careful when driving a motor vehicle or operating machinery
■ alcohol, sedatives, and tranquilizers may increase the drowsiness effect
Directions
To prevent motion sickness take it at least one hour before traveling
Adults and children 12 years of age and over: Oral dosage is 1 to 2 strips (25 to 50 mg) to dissolve on tongue once daily, or as directed by a physician.
| CVS MOTION SICKNESS STRIPS
meclizine hydrochloride film, soluble |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Tsukioka Film Pharma Co., Ltd. (693381774) |