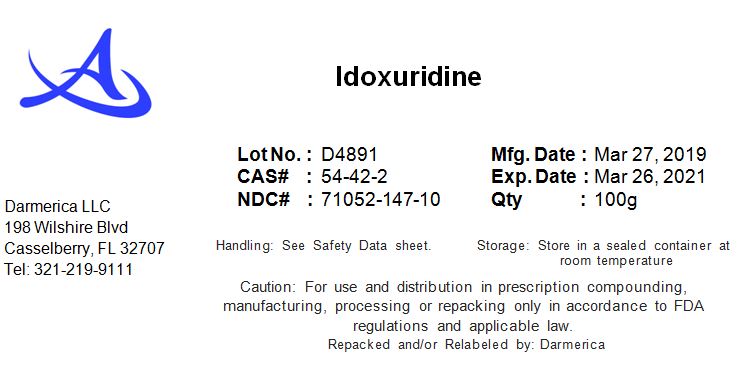
IDOXURUDINE- idoxurudine powder
Darmerica, LLC
----------
Idoxuridine
| IDOXURUDINE
idoxurudine powder |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Darmerica, LLC (080233052) |
Revised: 12/2021
Document Id: d2a64c7f-5c2c-2845-e053-2a95a90a181b
Set id: 5825d1fd-20ed-fda7-e053-2a91aa0a6585
Version: 4
Effective Time: 20211208
Darmerica, LLC