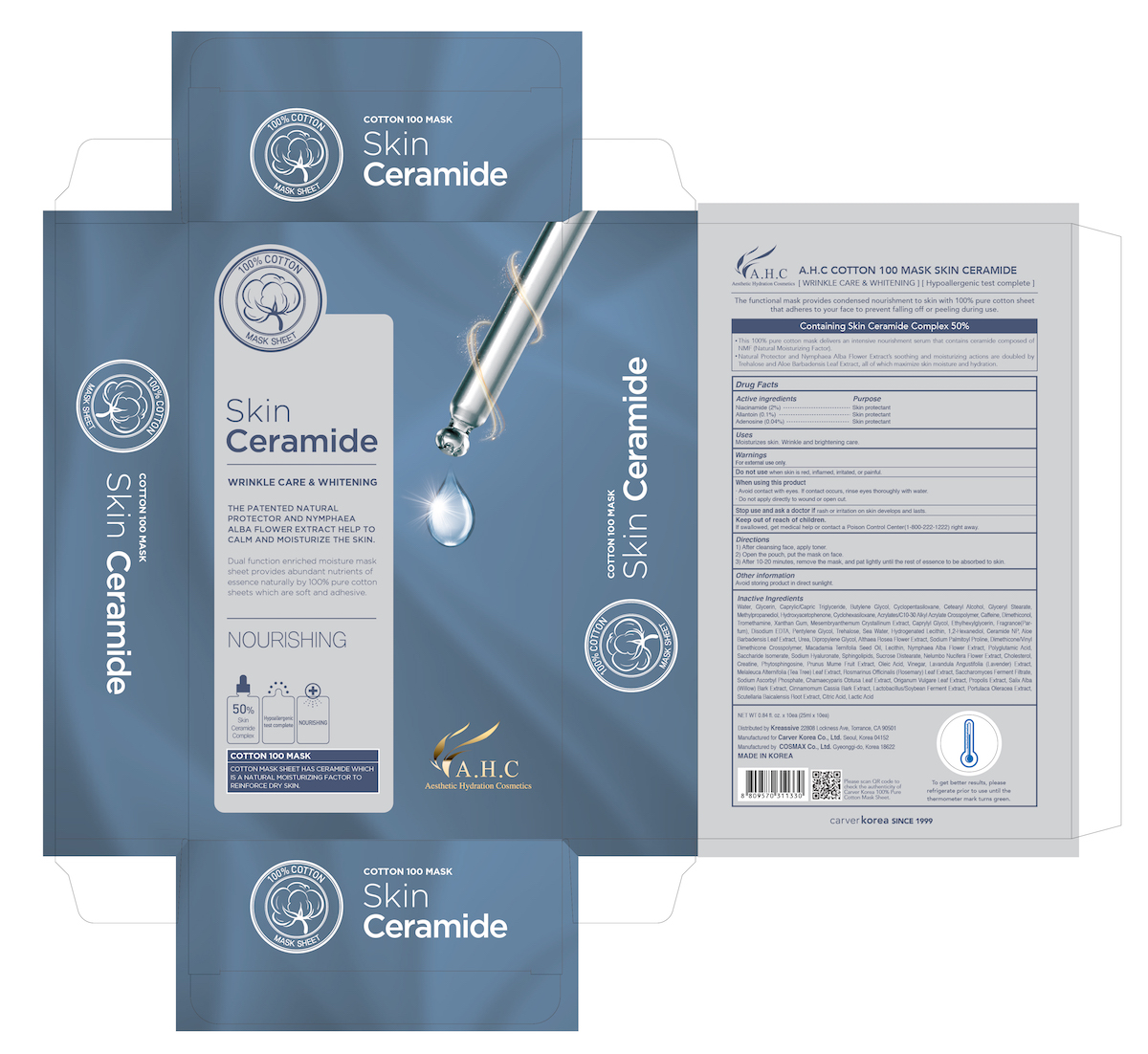
A.H.C COTTON 100 MASK SKIN CERAMIDE- niacinamide, allantoin, adenosine patch
Carver Korea Co.,Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
A.H.C COTTON 100 MASK SKIN CERAMIDE
Warnings
For external use only
Do not use when skin is red, inflamed, irritated, or painful
When using this produdct
- Avoid contact with eyes. If contact occurs, rinse eyes toroughly with water.
- Do not apply directly to wound or open cut.
Stop use and ask a doctor if rash or irritation on skin developes and lasts.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
1) After cleansing face, apply toner
2) Open the pouch, put the mask on face.
3) After 10-20 minutes, remove the mask, and pat lightly until the rest of essence to be absorbed to skin.
Inactive ingredients
Water, Glycerin, Caprylic/Capric Triglyceride, Butylene Glycol, Cyclopentasiloxane, Cetearyl Alcohol, Glyceryl Stearate, Methylpropanediol, Hydroxyacetophenone, Cyclohexasiloxane, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caffeine, Dimethiconol, Tromethamine, Xanthan Gum, Mesembryanthemum Crystallinum Extract, Caprylyl Glycol, Ethylhexylglycerin, Fragrance(Parfum), Disodium EDTA, Pentylene Glycol, Trehalose, Sea Water, Hydrogenated Lecithin, 1,2-Hexanediol, Ceramide NP, Aloe Barbadensis Leaf Extract, Urea, Dipropylene Glycol, Althaea Rosea Flower Extract, Sodium Palmitoyl Proline, Dimethicone/Vinyl Dimethicone Crosspolymer, Macadamia Ternifolia Seed Oil, Lecithin, Nymphaea Alba Flower Extract, Polyglutamic Acid, Saccharide Isomerate, Sodium Hyaluronate, Sphingolipids, Sucrose Distearate, Nelumbo Nucifera Flower Extract, Cholesterol, Creatine, Phytosphingosine, Prunus Mume Fruit Extract, Oleic Acid, Vinegar, Lavandula Angustifolia (Lavender) Extract, Melaleuca Alternifolia (Tea Tree) Leaf Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Saccharomyces Ferment Filtrate, Sodium Ascorbyl Phosphate, Chamaecyparis Obtusa Leaf Extract, Origanum Vulgare Leaf Extract, Propolis Extract, Salix Alba (Willow) Bark Extract, Cinnamomum Cassia Bark Extract, Lactobacillus/Soybean Ferment Extract, Portulaca Oleracea Extract, Scutellaria Baicalensis Root Extract, Citric Acid
| A.H.C COTTON 100 MASK SKIN CERAMIDE
niacinamide, allantoin, adenosine patch |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Carver Korea Co.,Ltd. (688442290) |
| Registrant - Carver Korea Co.,Ltd. (688442290) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cosmax, Inc. | 689049693 | manufacture(58930-105) | |