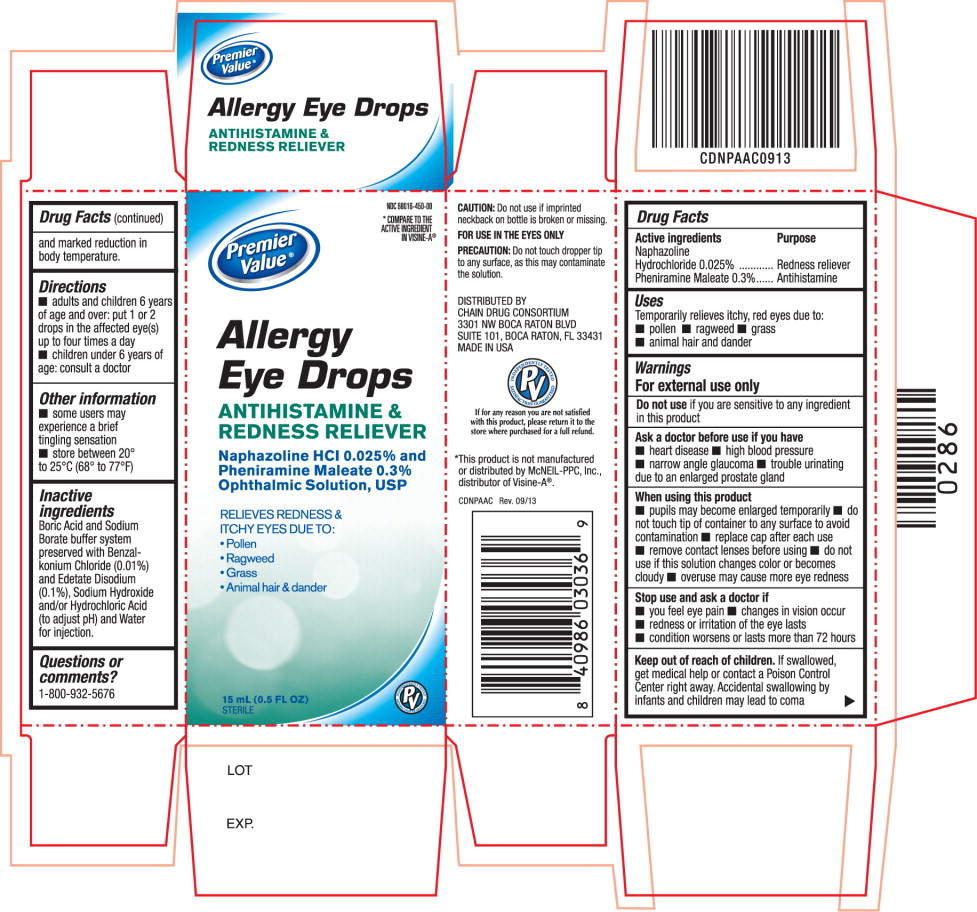
ALLERGY EYE DROPS- naphazoline hydrochloride and pheniramine maleate solution/ drops
Chain Drug Consortium
----------
Warnings
For external use only
Ask a doctor before use if you have
- heart disease
- high blood pressure
- narrow angle glaucoma
- trouble urinating due to an enlarged prostate gland
When using this product
- pupils may become enlarged temporarily
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- overuse may cause more eye redness
Directions
- adults and children 6 years of age and over: put 1 or 2 drops in the affected eye(s) up to four times a day
- children under 6 years of age: consult a doctor
Other information
- some users may experience a brief tingling sensation
- store between 20° to 25°C (68° to 77°F)
Inactive ingredients
Boric Acid and Sodium Borate buffer system preserved with Benzalkonium Chloride (0.01%) and Edetate Disodium (0.1%), Sodium Hydroxide and/or Hydrochloric Acid (to adjust pH) and Water for injection.
Principal Display Panel Text for Container Label:
NDC 68016-450-00
Premier
Value® Logo
Allergy
Eye Drops
ANTIHISTAMINE &
REDNESS RELIEVER
Naphazoline HCl 0.025% and
Pheniramine Maleate 0.3%
Ophthalmic Solution, USP
RELIEVES REDNESS
& ITCHY EYES
15 mL (0.5 FL OZ) STERILE
Principal Display Panel Text for Carton Label:
NDC 68016-450-00
*COMPARE TO THE
ACTIVE INGREDIENT
IN VISINE-A®
Premier
Value® Logo
Allergy
Eye Drops
ANTIHISTAMINE &
REDNESS RELIEVER
Naphazoline HCl 0.025% and
Pheniramine Maleate 0.3%
Ophthalmic Solution, USP
RELIEVES REDNESS &
ITCHY EYES DUE TO:
- Pollen
- Ragweed
- Grass
- Animal hair & dander
15 mL (0.5 FL OZ)
STERILE PV Brand Logo
| ALLERGY EYE DROPS
naphazoline hydrochloride and pheniramine maleate solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Chain Drug Consortium (101668460) |