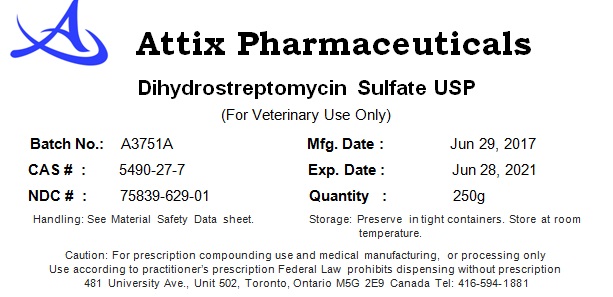
DIHYDROSTREPTOMYCIN SULFATE- dihydrostreptomycin sulfate powder
Attix Pharmaceuticals
----------
Dihydrostreptomycin Sulfate
| DIHYDROSTREPTOMYCIN SULFATE
dihydrostreptomycin sulfate powder |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Attix Pharmaceuticals (248276599) |
Revised: 12/2021
Document Id: d2927510-f5ad-bdb7-e053-2a95a90a42b2
Set id: 5786085c-e62b-3e42-e053-2991aa0a269d
Version: 5
Effective Time: 20211207
Attix Pharmaceuticals