Label: ZOMIG- zolmitriptan tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 68258-7103-3 - Packager: Dispensing Solutions, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0310-0211
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 10, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
ZOMIG® (zolmitriptan) Tablets and ZOMIG-ZMT® (zolmitriptan) Orally Disintegrating Tablets contain zolmitriptan, which is a selective 5-hydroxytryptamine 1B/1D (5-HT1B/1D) receptor agonist. Zolmitriptan is chemically designated as (S)-4-[[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]methyl]-2-oxazolidinone and has the following chemical structure:
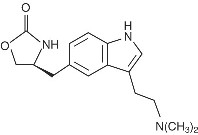
The empirical formula is C16H21N3O2, representing a molecular weight of 287.36. Zolmitriptan is a white to almost white powder that is readily soluble in water. ZOMIG Tablets are available as 2.5 mg (yellow) and 5 mg (pink) film coated tablets for oral administration. The film coated tablets contain anhydrous lactose NF, microcrystalline cellulose NF, sodium starch glycolate NF, magnesium stearate NF, hydroxypropyl methylcellulose USP, titanium dioxide USP, polyethylene glycol 400 NF, yellow iron oxide NF (2.5 mg tablet), red iron oxide NF (5 mg tablet), and polyethylene glycol 8000 NF.
ZOMIG-ZMT® Orally Disintegrating Tablets are available as 2.5 mg and 5.0 mg white uncoated tablets for oral administration. The orally disintegrating tablets contain mannitol USP, microcrystalline cellulose NF, crospovidone NF, aspartame NF, sodium bicarbonate USP, citric acid anhydrous USP, colloidal silicon dioxide NF, magnesium stearate NF and orange flavor SN 027512.
-
CLINICAL PHARMACOLOGY
Mechanism of Action:
Zolmitriptan binds with high affinity to human recombinant 5-HT1D and 5-HT1B receptors. Zolmitriptan exhibits modest affinity for 5-HT1A receptors, but has no significant affinity (as measured by radioligand binding assays) or pharmacological activity at 5-HT2, 5-HT3, 5-HT4, alpha1-, alpha2-, or beta1- adrenergic; H1, H2, histaminic; muscarinic; dopamine1, or dopamine2 receptors. The N-desmethyl metabolite also has high affinity for 5-HT1B/1D and modest affinity for 5-HT1A receptors.
Current theories proposed to explain the etiology of migraine headache suggest that symptoms are due to local cranial vasodilatation and/or to the release of sensory neuropeptides (vasoactive intestinal peptide, substance P and calcitonin gene-related peptide) through nerve endings in the trigeminal system. The therapeutic activity of zolmitriptan for the treatment of migraine headache can most likely be attributed to the agonist effects at the 5-HT1B/1D receptors on intracranial blood vessels (including the arterio-venous anastomoses) and sensory nerves of the trigeminal system which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
Clinical Pharmacokinetics and Bioavailability
Absorption:
Zolmitriptan is well absorbed after oral administration for both the conventional tablets and the orally disintegrating tablets. Zolmitriptan displays linear kinetics over the dose range of 2.5 to 50 mg.
The AUC and Cmax of zolmitriptan are similar following administration of ZOMIG Tablets and ZOMIG-ZMT Orally Disintegrating Tablets, but the Tmax is somewhat later with ZOMIG-ZMT, with a median Tmax of 3 hours for the orally disintegrating tablet compared with 1.5 hours for the conventional tablet. The AUC, Cmax,and Tmax for the active N-desmethyl metabolite are similar for the two formulations.
During a moderate to severe migraine attack, mean AUC0-4 and Cmax for zolmitriptan, dosed as a conventional tablet, were decreased by 40% and 25%, respectively, and mean Tmax was delayed by one-half hour compared to the same patients during a migraine free period.
Food has no significant effect on the bioavailability of zolmitriptan. No accumulation occurred on multiple dosing.
Distribution:
Mean absolute bioavailability is approximately 40%. The mean apparent volume of distribution is 7.0 L/kg. Plasma protein binding of zolmitriptan is 25% over the concentration range of 10- 1000ng/mL.
Metabolism:
Zolmitriptan is converted to an active N-desmethyl metabolite such that the metabolite concentrations are about two-thirds that of zolmitriptan. Because the 5HT1B/1D potency of the metabolite is 2 to 6 times that of the parent, the metabolite may contribute a substantial portion of the overall effect after zolmitriptan administration.
Elimination:
Total radioactivity recovered in urine and feces was 65% and 30% of the administered dose, respectively. About 8% of the dose was recovered in the urine as unchanged zolmitriptan. Indole acetic acid metabolite accounted for 31% of the dose, followed by N-oxide (7%) and N-desmethyl (4%) metabolites. The indole acetic acid and N-oxide metabolites are inactive.
Mean total plasma clearance is 31.5mL/min/kg, of which one-sixth is renal clearance. The renal clearance is greater than the glomerular filtration rate suggesting renal tubular secretion.
Special Populations:
Age: Zolmitriptan pharmacokinetics in healthy elderly non-migraineur volunteers (age 65−76 yrs) were similar to those in younger non-migraineur volunteers (age 18 - 39 yrs).
Gender: Mean plasma concentrations of zolmitriptan were up to 1.5-fold higher in females than males.
Renal Impairment: Clearance of zolmitriptan was reduced by 25% in patients with severe renal impairment (Clcr ≥ 5 ≤ 25 mL/min) compared to the normal group (Clcr > = 70 mL/min); no significant change in clearance was observed in the moderately renally impaired group (Clcr ≥ 26 ≤ 50 mL/min).
Hepatic Impairment: In severely hepatically impaired patients, the mean Cmax, Tmax, and AUC0-∞ of zolmitriptan were increased 1.5, 2 (2 vs 4 hr), and 3-fold, respectively, compared to normals. Seven out of 27 patients experienced 20 to 80 mm Hg elevations in systolic and/or diastolic blood pressure after a 10 mg dose. Zolmitriptan should be administered with caution in subjects with liver disease, generally using doses less than 2.5 mg (see WARNINGS and PRECAUTIONS).
Hypertensive Patients: No differences in the pharmacokinetics of zolmitriptan or its effects on blood pressure were seen in mild to moderate hypertensive volunteers compared to normotensive controls.
Race:: Retrospective analysis of pharmacokinetic data between Japanese and Caucasians revealed no significant differences.
Drug Interactions:
All drug interaction studies were performed in healthy volunteers using a single 10 mg dose of zolmitriptan and a single dose of the other drug except where otherwise noted.
Fluoxetine: The pharmacokinetics of zolmitriptan, as well as its effect on blood pressure, were unaffected by 4 weeks of pretreatment with oral fluoxetine (20 mg/day).
MAO Inhibitors: Following one week of administration of 150 mg bid moclobemide, a specific MAO-A inhibitor, there was an increase of about 25% in both Cmax and AUC for zolmitriptan and a 3-fold increase in the Cmax and AUC of the active N-desmethyl metabolite of zolmitriptan (see CONTRAINDICATIONS and PRECAUTIONS).
Selegiline, a selective MAO-B inhibitor, at a dose of 10 mg/day for 1 week, had no effect on the pharmacokinetics of zolmitriptan and its metabolite.
Propranolol: Cmax and AUC of zolmitriptan increased 1.5-fold after one week of dosing with propranolol (160 mg/day). Cmax and AUC of the N-desmethyl metabolite were reduced by 30% and 15%, respectively. There were no interactive effects on blood pressure or pulse rate following administration of propranolol with zolmitriptan.
Acetaminophen: A single 1 g dose of acetaminophen does not alter the pharmacokinetics of zolmitriptan and its N-desmethyl metabolite. However, zolmitriptan delayed the Tmax of acetaminophen by one hour.
Metoclopramide: A single 10 mg dose of metoclopramide had no effect on the pharmacokinetics of zolmitriptan or its metabolites.
Oral Contraceptives: Retrospective analysis of pharmacokinetic data across studies indicated that mean plasma concentrations of zolmitriptan were generally higher in females taking oral contraceptives compared to those not taking oral contraceptives. Mean Cmax and AUC of zolmitriptan were found to be higher by 30% and 50%, respectively, and Tmax was delayed by one-half hour in females taking oral contraceptives. The effect of zolmitriptan on the pharmacokinetics of oral contraceptives has not been studied.
Cimetidine: Following the administration of cimetidine, the half-life and AUC of a 5 mg dose of zolmitriptan and its active metabolite were approximately doubled (see PRECAUTIONS).
Clinical Studies:
The efficacy of ZOMIG Tablets in the acute treatment of migraine headaches was demonstrated in five randomized, double-blind, placebo controlled studies, of which 2 utilized the 1 mg dose, 2 utilized the 2.5 mg dose and 4 utilized the 5 mg dose; all studies used the marketed formulation. In study 1, patients treated their headaches in a clinic setting. In the other studies, patients treated their headaches as outpatients. In study 4, patients who had previously used sumatriptan were excluded, whereas in the other studies no such exclusion was applied. Patients enrolled in these 5 studies were predominantly female (82%) and Caucasian (97%) with a mean age of 40 years (range 12-65). Patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed at 1, 2, and, in most studies, 4 hours after dosing. Associated symptoms such as nausea, photophobia, and phonophobia were also assessed. Maintenance of response was assessed for up to 24 hours postdose. A second dose of ZOMIG Tablets or other medication was allowed 2 to 24 hours after the initial treatment for persistent and recurrent headache. The frequency and time to use of these additional treatments were also recorded. In all studies, the effect of zolmitriptan was compared to placebo in the treatment of a single migraine attack.
In all five studies, the percentage of patients achieving headache response 2 hours after treatment was significantly greater among patients receiving ZOMIG Tablets at all doses (except for the 1 mg dose in the smallest study) compared to those who received placebo. In the two studies that evaluated the 1 mg dose, there was a statistically significant greater percentage of patients with headache response at 2 hours in the higher dose groups (2.5 and/or 5 mg) compared to the 1 mg dose group. There were no statistically significant differences between the 2.5 and 5 mg dose groups (or of doses up to 20 mg) for the primary end point of headache response at 2 hours in any study. The results of these controlled clinical studies are summarized in Table 1.
Comparisons of drug performance based upon results obtained in different clinical trials are never reliable. Because studies are conducted at different times, with different samples of patients, by different investigators, employing different criteria and/or different interpretations of the same criteria, under different conditions (dose, dosing regimen, etc.), quantitative estimates of treatment response and the timing of response may be expected to vary considerably from study to study.
Table 1: Percentage of Patients with Headache Response (Mild or no Headache) 2 Hours Following Treatment (n=number of patients randomized). Placebo
ZOMIG
1.0 mg
ZOMIG
2.5 mg
ZOMIG
5 mg
Study 1
16%
(n=19)
27%
(n=22)
NA*
(n=20)
Study 2
19%
(n=88)
NA*
NA*
66%†
(n=179)
Study 3
34%
(n=121)
50%†
(n=140)
(n=260)
(n=245)
Study 4
44%
(n=55)
NA*
NA*
59%†
(n=491)
Study 5
36%
(n=92)
NA*
NA*
62%†
(n=178)
NA*
The estimated probability of achieving an initial headache response by 4 hours following treatment is depicted in Figure 1.
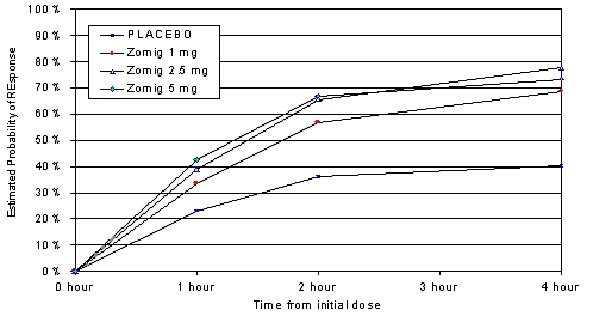
Figure 1: Estimated Probability Of Achieving Initial Headache ResponseWithin 4 Hours*
*Figure 1 shows the Kaplan-Meier plot of the probability over time of obtaining headache response (no or mild pain) following treatment with zolmitriptan. The averages displayed are based on pooled data from 3 placebo controlled, outpatient trials providing evidence of efficacy (Trials 2, 3 and 5). Patients not achieving headache response or taking additional treatment prior to 4 hours were censored at 4 hours.
For patients with migraine associated photophobia, phonophobia, and nausea at baseline, there was a decreased incidence of these symptoms following administration of ZOMIG as compared to placebo.
Two to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
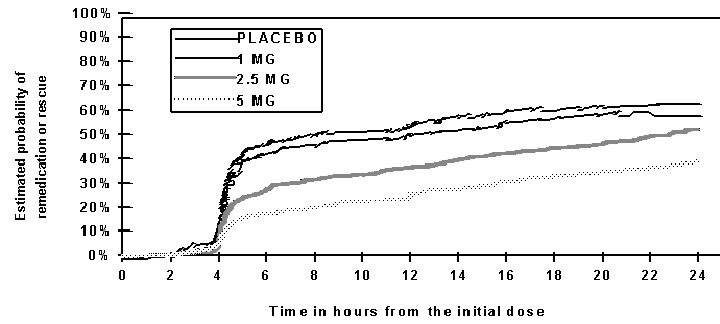
Figure 2: The Estimated Probability Of Patients Taking A Second Dose Or Other Medication For Migraines Over The 24 Hours Following The Initial Dose Of Study Treatment*
*This Kaplan-Meier plot is based on data obtained in 3 placebo controlled clinical trials (Study 2, 3 and 5). Patients not using additional treatments were censored at 24 hours. The plot includes both patients who had headache response at 2 hours and those who had no response to the initial dose. It should be noted that the protocols did not allow remedication within 2 hours postdose.
The efficacy of ZOMIG was unaffected by presence of aura; duration of headache prior to treatment; relationship to menses; gender, age, or weight of the patient; pretreatment nausea, or concomitant use of common migraine prophylactic drugs.
ZOMIG-ZMT Orally Disintegrating Tablets
The efficacy of ZOMIG-ZMT 2.5 mg was demonstrated in a randomized, placebo-controlled trial that was similar in design to the trials of ZOMIG Tablets. Patients were instructed to treat a moderate to severe headache. Of the 471 patients treated in the study, 87% were female and 97% were Caucasian, with a mean age of 41 years (range 18-62). At 2 hours post-dosing response rates in patients treated with ZOMIG-ZMT 2.5 mg were 63% compared to 22% in the placebo group. The difference was statistically significant. The estimated probability of achieving an initial headache response by 2 hours following treatment with ZOMIG-ZMT Tablets is depicted in Figure 3.
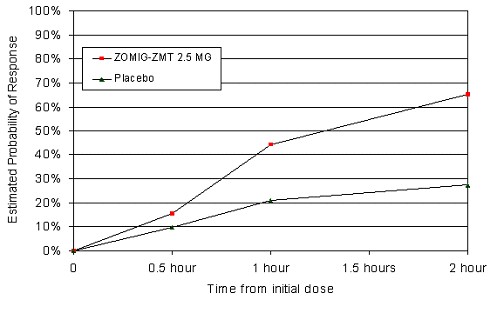
Figure 3: Estimated Probability of Achieving Initial Headache Response by 2 Hours
Figure 3 shows the Kaplan-Meier plot of the probability over time of obtaining headache response (no or mild pain) following treatment with ZOMIG-ZMT Tablets or placebo. Patients taking additional treatment or not achieving headache response prior to 2 hours were censored at 2 hours.
For patients with migraine-associated photophobia, phonophobia and nausea at baseline, there was a decreased incidence of these symptoms following administration of ZOMIG-ZMT as compared to placebo.
Two to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 4.
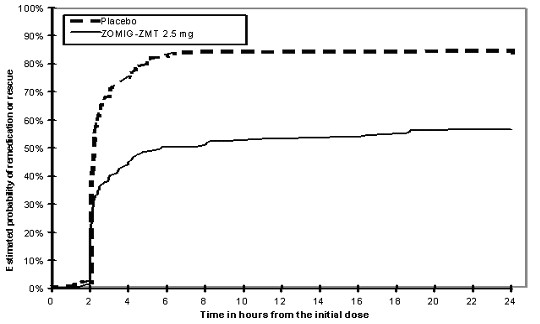
Figure 4: The Estimated Probability of Patients Taking a Second Dose or Other Medication for Migraines Over the 24 Hours Following The Initial Dose of Study Treatment
In this Kaplan-Meier plot, patients not using additional treatments were censored at 24 hours. The plot includes both patients who had headache response at 2 hours and those who had no response to the initial dose. Remedication was allowed 2 hours post-dose, and unlike the conventional tablet, remedication prior to 4 hours was not discouraged.
-
INDICATIONS AND USAGE
ZOMIG is indicated for the acute treatment of migraine with or without aura in adults.
ZOMIG should only be used where a clear diagnosis of migraine has been established.
ZOMIG is not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine (see CONTRAINDICATIONS). Safety and effectiveness of ZOMIG have not been established for cluster headache, which is present in an older, predominantly male population.
-
CONTRAINDICATIONS
ZOMIG should not be given to patients with ischemic heart disease (angina pectoris, history of myocardial infarction, or documented silent ischemia) or to patients who have symptoms or findings consistent with ischemic heart disease, coronary artery vasospasm, including Prinzmetal’s variant angina, or other significant underlying cardiovascular disease (see WARNINGS and PRECAUTIONS).
ZOMIG should not be given to patients with cerebrovascular syndromes including (but not limited to) stroke of any type as well as transient ischemic attacks (see WARNINGS).
ZOMIG should not be given to patients with peripheral vascular disease including (but not limited to) ischemic bowel disease (see WARNINGS and PRECAUTIONS).
Because ZOMIG may increase blood pressure, it should not be given to patients with uncontrolled hypertension (see WARNINGS).
ZOMIG should not be used within 24 hours of treatment with another 5-HT1 agonist, or an ergotamine-containing or ergot-type medication like dihydroergotamine or methysergide.
ZOMIG should not be administered to patients with hemiplegic or basilar migraine.
Concurrent administration of MAO-A inhibitors or use of zolmitriptan within 2 weeks of discontinuation of MAO-A inhibitor therapy is contraindicated (see CLINICAL PHARMACOLOGY: Drug Interactions and PRECAUTIONS: Drug Interactions).
ZOMIG is contraindicated in patients who are hypersensitive to zolmitriptan or any of its inactive ingredients.
-
WARNINGS
Risk of Myocardial Ischemia and/or Infarction and Other Adverse Cardiac Events: ZOMIG should not be given to patients with documented ischemic or vasospastic coronary artery disease (see CONTRAINDICATIONS). It is strongly recommended that zolmitriptan not be given to patients in whom unrecognized coronary artery disease (CAD) is predicted by the presence of risk factors (e.g., hypertension, hypercholesterolemia, smoker, obesity, diabetes, strong family history of CAD, female with surgical or physiological menopause, or male over 40 years of age) unless a cardiovascular evaluation provides satisfactory clinical evidence that the patient is reasonably free of coronary artery and ischemic myocardial disease or other significant underlying cardiovascular disease. The sensitivity of cardiac diagnostic procedures to detect cardiovascular disease or predisposition to coronary artery vasospasm is modest, at best. If, during the cardiovascular evaluation, the patient’s medical history, electrocardiographic or other investigations reveal findings indicative of, or consistent with, coronary artery vasospasm or myocardial ischemia, zolmitriptan should not be administered (see CONTRAINDICATIONS). For patients with risk factors predictive of CAD, who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of zolmitriptan take place in the setting of a physician’s office or similar medically staffed and equipped facility unless the patient has previously received zolmitriptan. Because cardiac ischemia can occur in the absence of clinical symptoms, consideration should be given to obtaining on the first occasion of use an electrocardiogram (ECG) during the interval immediately following ZOMIG, in these patients with risk factors.
It is recommended that patients who are intermittent long-term users of ZOMIG and who have or acquire risk factors predictive of CAD, as described above, undergo periodic interval cardiovascular evaluation as they continue to use ZOMIG.
The systematic approach described above is intended to reduce the likelihood that patients with unrecognized cardiovascular disease will be inadvertently exposed to zolmitriptan.
Cardiac Events and Fatalities:
Serious adverse cardiac events, including acute myocardial infarction, have been reported within a few hours following administration of zolmitriptan. Life-threatening disturbances of cardiac rhythm, and death have been reported within a few hours following the administration of other 5-HT1 agonists. Considering the extent of use of 5-HT1 agonists in patients with migraine, the incidence of these events is extremely low.
ZOMIG can cause coronary vasospasm; at least one of these events occurred in a patient with no cardiac disease history and with documented absence of coronary artery disease. Because of the close proximity of the events to ZOMIG use, a causal relationship cannot be excluded. In the cases where there has been known underlying coronary artery disease, the relationship is uncertain.
Patients with symptomatic Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders should not receive ZOMIG.
Premarketing experience with zolmitriptan:
Among the more than 2,500 patients with migraine who participated in premarketing controlled clinical trials of ZOMIG Tablets, no deaths or serious cardiac events were reported.
Postmarketing experience with zolmitriptan:
Serious cardiovascular events have been reported in association with the use of ZOMIG Tablets, and in very rare cases, these events have occurred in the absence of known cardiovascular disease. The uncontrolled nature of postmarketing surveillance, however, makes it impossible to determine definitively the proportion of the reported cases that were actually caused by zolmitriptan or to reliably assess causation in individual cases.
Cerebrovascular Events and Fatalities with 5-HT1 agonists:
Cerebral hemorrhage, subarachnoid hemorrhage, stroke, and other cerebrovascular events have been reported in patients treated with 5-HT1 agonists; and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. It should be noted that patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, transient ischemic attack). (See CONTRAINDICATIONS.)
Serotonin Syndrome:
The development of a potentially life-threatening serotonin syndrome may occur with triptans, including ZOMIG treatment, particularly during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs). If concomitant treatment with ZOMIG and an SSRI (e.g. fluoxetine, paroxetine, sertraline, fluvoxamine, citalopram, escitalopram) or SNRI (e.g., venlafaxine, duloxetine) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g. hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g. nausea, vomiting, diarrhea). (See PRECAUTIONS, Drug Interactions).
Other Vasospasm-Related Events:
5-HT1 agonists may cause vasospastic reactions other than coronary artery vasospasm such as peripheral and gastrointestinal vascular ischemia. As with other serotonin 5HT1 agonists, very rare gastrointestinal ischemic events including ischemic colitis and gastrointestinal infarction or necrosis have been reported with ZOMIG Tablets; these may present as bloody diarrhea or abdominal pain. (See CONTRAINDICATIONS.)
Very rare reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5- HT1 agonists. Visual disorders may also be part of a migraine attack.
Increase in Blood Pressure:
As with other 5-HT1 agonists, significant elevations in systemic blood pressure have been reported on rare occasions with ZOMIG Tablet use, in patients with and without a history of hypertension; very rarely these increases in blood pressure have been associated with significant clinical events. Zolmitriptan is contraindicated in patients with uncontrolled hypertension. In volunteers, an increase of 1 and 5 mm Hg in the systolic and diastolic blood pressure, respectively, was seen at 5 mg. In the headache trials, vital signs were measured only in the small inpatient study and no effect on blood pressure was seen. In a study of patients with moderate to severe liver disease, 7 of 27 experienced 20 to 80 mm Hg elevations in systolic and/or diastolic blood pressure after a dose of 10 mg of zolmitriptan (see CONTRAINDICATIONS).
An 18% increase in mean pulmonary artery pressure was seen following dosing with another 5-HT1 agonist in a study evaluating subjects undergoing cardiac catheterization.
-
PRECAUTIONS
General:
As with other 5-HT1B/1D agonists, sensations of tightness, pain, pressure, and heaviness have been reported after treatment with ZOMIG Tablets in the precordium, throat, neck, and jaw. Because zolmitriptan may cause coronary artery vasospasm, patients who experience signs or symptoms suggestive of angina following dosing should be evaluated for the presence of CAD or a predisposition to Prinzmetal’s variant angina before receiving additional doses of medication, and should be monitored electrocardiographically if dosing is resumed and similar symptoms recur. Similarly, patients who experience other symptoms or signs suggestive of decreased arterial flow, such as ischemic bowel syndrome or Raynaud’s syndrome following the use of any 5-HT1 agonist are candidates for further evaluation. (see CONTRAINDICATIONS and WARNINGS.)
Zolmitriptan should also be administered with caution to patients with diseases that may alter the absorption, metabolism, or excretion of drugs, such as impaired hepatic function (see CLINICAL PHARMACOLOGY).
For a given attack, if a patient does not respond to the first dose of zolmitriptan, the diagnosis of migraine headache should be reconsidered before administration of a second dose.
Binding to Melanin-Containing Tissues:
When pigmented rats were given a single oral dose of 10 mg/kg of radiolabeled zolmitriptan, the radioactivity in the eye after 7 days, the latest time point examined, was still 75% of the value measured after 4 hours. This suggests that zolmitriptan and/or its metabolites may bind to the melanin of the eye. Because there could be accumulation in melanin rich tissues over time, this raises the possibility that zolmitriptan could cause toxicity in these tissues after extended use. However, no effects on the retina related to treatment with zolmitriptan were noted in any of the toxicity studies. Although no systematic monitoring of ophthalmologic function was undertaken in clinical trials, and no specific recommendations for ophthalmologic monitoring are offered, prescribers should be aware of the possibility of long-term ophthalmologic effects.
Phenylketonurics:
Phenylketonuric patients should be informed that ZOMIG-ZMT contain phenylalanine (a component of aspartame). Each 2.5 mg orally disintegrating tablet contains 2.81 mg phenylalanine. Each 5 mg orally disintegrating tablet contains 5.62 mg phenylalanine.
Information for Patients
See PATIENT INFORMATION at the end of this labeling for the text of the separate leaflet provided for patients.
ZOMIG-ZMT Orally Disintegrating Tablets
The orally disintegrating tablet is packaged in a blister. Patients should be instructed not to remove the tablet from the blister until just prior to dosing. The blister pack should then be peeled open, and the orally disintegrating tablet placed on the tongue, where it will dissolve and be swallowed with the saliva.
Patients should be cautioned about the risk of serotonin syndrome with the use of ZOMIG or other triptans, especially during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs).
Drug Interactions
Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because there is a theoretical basis that these effects may be additive, use of ergotamine-containing or ergot-type medications (like dihydroergotamine or methysergide) and zolmitriptan within 24 hours of each other should be avoided (see CONTRAINDICATIONS).
MAO-A inhibitors increase the systemic exposure of zolmitriptan. Therefore, the use of zolmitriptan in patients receiving MAO-A inhibitors is contraindicated (see CLINICAL PHARMACOLOGYand CONTRAINDICATIONS).
Concomitant use of other 5-HT1B/1D agonists within 24 hours of ZOMIG treatment is not recommended. (see CONTRAINDICATIONS).
Following administration of cimetidine, the half-life and AUC of zolmitriptan and its active metabolites were approximately doubled (see CLINICAL PHARMACOLOGY).
Selective Serotonin Reuptake Inhibitors/Serotonin Norepinephrine Reuptake Inhibitors and Serotonin Syndrome: Cases of life-threatening serotonin syndrome have been reported during combined use of selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) and triptans (See WARNINGS).
Drug/Laboratory Test Interactions:
Zolmitriptan is not known to interfere with commonly employed clinical laboratory tests.
Carcinogenesis:
Carcinogenicity studies by oral gavage were carried out in mice and rats at doses up to 400 mg/kg/day. Mice were dosed for 85 weeks (males) and 92 weeks (females). The exposure (plasma AUC of parent drug) at the highest dose level was approximately 800 times that seen in humans after a single 10 mg dose (the maximum recommended total daily dose). There was no effect of zolmitriptan on tumor incidence. Control, low dose, and middle dose rats were dosed for 104-105 weeks; the high dose group was sacrificed after 101 weeks (males) and 86 weeks (females) due to excess mortality. Aside from an increase in the incidence of thyroid follicular cell hyperplasia and thyroid follicular cell adenomas seen in male rats receiving 400 mg/kg/day, an exposure approximately 3000 times that seen in humans after dosing with 10 mg, no tumors were noted.
Mutagenesis:
Zolmitriptan was mutagenic in an Ames test, in 2 of 5 strains of S. typhimurium tested, in the presence of, but not in the absence of, metabolic activation. It was not mutagenic in an in vitro mammalian gene cell mutation (CHO/HGPRT) assay. Zolmitriptan was clastogenic in an in vitro human lymphocyte assay both in the absence of and the presence of metabolic activation; it was not clastogenic in an in vivo mouse micronucleus assay. It was also not genotoxic in an unscheduled DNA synthesis study.
Impairment of Fertility:
Studies of male and female rats administered zolmitriptan prior to and during mating and up to implantation have shown no impairment of fertility at doses up to 400 mg/kg/day. Exposure at this dose was approximately 3000 times exposure at the maximum recommended human dose of 10 mg/day.
Pregnancy:
Pregnancy Category C:
There are no adequate and well controlled studies in pregnant women; therefore, zolmitriptan should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In reproductive toxicity studies in rats and rabbits, oral administration of zolmitriptan to pregnant animals was associated with embryolethality and fetal abnormalities. When pregnant rats were administered oral zolmitriptan during the period of organogenesis at doses of 100, 400, and 1200 mg/kg/day, there was a dose-related increase in embryolethality which became statistically significant at the high dose. The maternal plasma exposures at these doses were approximately 280, 1100, and 5000 times the exposure in humans receiving the maximum recommended total daily dose of 10 mg. The high dose was maternally toxic, as evidenced by a decreased maternal body weight gain during gestation. In a similar study in rabbits, embryolethality was increased at the maternally toxic doses of 10 and 30 mg/kg/day (maternal plasma exposures equivalent to 11 and 42 times exposure in humans receiving the maximum recommended total daily dose of 10 mg), and increased incidences of fetal malformations (fused sternebrae, rib anomalies) and variations (major blood vessel variations, irregular ossification pattern of ribs) were observed at 30 mg/kg/day. Three mg/kg/day was a no effect dose (equivalent to human exposure at a dose of 10 mg). When female rats were given zolmitriptan during gestation, parturition, and lactation, an increased incidence of hydronephrosis was found in the offspring at the maternally toxic dose of 400 mg/kg/day (1100 times human exposure).
Nursing Mothers:
It is not known whether zolmitriptan is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when zolmitriptan is administered to a nursing woman. Lactating rats dosed with zolmitriptan had milk levels equivalent to maternal plasma levels at 1 hour and 4 times higher than plasma levels at 4 hours.
Pediatric Use:
Safety and effectiveness of ZOMIG Tablets in pediatric patients have not been established. Therefore, ZOMIG is not recommended for use in patients under 18 years of age.
One randomized, placebo-controlled clinical trial evaluating zolmitriptan tablets (2.5, 5 and 10 mg) in pediatric patients aged 12-17 years evaluated a total of 696 adolescent migraineurs. This study did not establish the efficacy of zolmitriptan compared to placebo in the treatment of migraine in adolescents. Adverse events observed were similar in nature and frequency to those reported in clinical trials in adults.
Postmarketing experience with ZOMIG and other triptans includes a limited number of reports that describe pediatric patients who have experienced clinically serious adverse events that are similar in nature to those reported rarely in adults.
Geriatric Use:
Although the pharmacokinetic disposition of the drug in the elderly is similar to that seen in younger adults, there is no information about the safety and effectiveness of zolmitriptan in this population because patients over age 65 were excluded from the controlled clinical trials. (see CLINICAL PHARMACOLOGY: Special Populations)
-
ADVERSE REACTIONS:
Serious cardiac events, including myocardial infarction, have occurred following the use of ZOMIG Tablets. These events are extremely rare and most have been reported in patients with risk factors predictive of CAD. Events reported, in association with drugs of this class, have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation (see CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS).
Incidence in Controlled Clinical Trials:
Among 2,633 patients treated with ZOMIG Tablets in the active and placebo controlled trials, no patients withdrew for reasons related to adverse events, but as patients treated a single headache in these trials, the opportunity for discontinuation was limited. In a long-term, open label study where patients were allowed to treat multiple migraine attacks for up to 1 year, 8% (167 out of 2,058) withdrew from the trial because of adverse experience. The most common events were paresthesia, asthenia, nausea, dizziness, pain, chest or neck tightness or heaviness, somnolence, and warm sensation.
Table 2 lists the adverse events that occurred in ≥ 2% of the 2,074 patients in any one of the ZOMIG 1 mg, ZOMIG 2.5 mg or ZOMIG 5 mg Tablets dose groups of the controlled clinical trials. Only events that were more frequent in a ZOMIG Tablets group compared to the placebo groups are included. The events cited reflect experience gained under closely monitored conditions of clinical trials in a highly selected patient population. In actual clinical practice or in other clinical trials, these frequency estimates may not apply, as the conditions of use, reporting behavior, and the kinds of patients treated may differ.
Several of the adverse events appear dose related, notably paresthesia, sensation of heaviness or tightness in chest, neck, jaw, and throat, dizziness, somnolence, and possibly asthenia and nausea.
Table 2: Adverse Experience Incidence in Five Placebo-Controlled Migraine Clinical Trials: Events Reported By ≥ 2% Patients Treated With ZOMIG Tablets Adverse Event Type
Placebo
(n=401)
ZOMIG
1 mg
(n=163)
ZOMIG
2.5 mg
(n=498)
ZOMIG
5 mg
(n=1012)
ATYPICAL SENSATIONS
6%
12%
12%
18%
Hyperesthesia
1%
1%
1%
2%
Paresthesia (all types)
2%
5%
7%
9%
Sensation warm/cold
4%
6%
5%
7%
PAIN AND PRESSURE SENSATIONS
7%
13%
14%
22%
Chest - pain/tightness/pressure and/or heaviness
1%
2%
3%
4%
Neck/throat/jaw - pain/tightness/pressure
3%
4%
7%
10%
Heaviness other than chest or neck
1%
1%
2%
5%
Pain − location specified
1%
2%
2%
3%
Other − Pressure/tightness/heaviness
0
2%
2%
2%
DIGESTIVE
8%
11%
16%
14%
Dry mouth
2%
5%
3%
3%
Dyspepsia
1%
3%
2%
1%
Dysphagia
0%
0%
0%
2%
Nausea
4%
4%
9%
6%
NEUROLOGICAL
10%
11%
17%
21%
Dizziness
4%
6%
8%
10%
Somnolence
3%
5%
6%
8%
Vertigo
0%
0%
0%
2%
OTHER
Asthenia
3%
5%
3%
9%
Palpitations
1%
0%
<1%
2%
Myalgia
<1%
1%
1%
2%
Myasthenia
<1%
0%
1%
2%
Sweating
1%
0%
2%
3%
ZOMIG is generally well tolerated. Across all doses, most adverse reactions were mild and transient and did not lead to long-lasting effects. The incidence of adverse events in controlled clinical trials was not affected by gender, weight, or age of the patients; use of prophylactic medications; or presence of aura. There were insufficient data to assess the impact of race on the incidence of adverse events.
Other Events:
In the paragraphs that follow, the frequencies of less commonly reported adverse clinical events are presented. Because the reports include events observed in open and uncontrolled studies, the role of ZOMIG in their causation cannot be reliably determined. Furthermore, variability associated with adverse event reporting, the terminology used to describe adverse events, etc., limit the value of the quantitative frequency estimates provided. Event frequencies are calculated as the number of patients who used ZOMIG Tablets (n=4,027) and reported an event divided by the total number of patients exposed to ZOMIG Tablets. All reported events are included except those already listed in the previous table, those too general to be informative, and those not reasonably associated with the use of the drug. Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: infrequent adverse events are those occurring in 1/100 to 1/1,000 patients and rare adverse events are those occurring in fewer than 1/1,000 patients.
Atypical sensation: Infrequent was hyperesthesia.
General: Infrequent were allergy reaction, chills, facial edema, fever, malaise, and photosensitivity.
Cardiovascular: Infrequent were arrhythmias, hypertension, and syncope. Rare were bradycardia, extrasystoles, postural hypotension, QT prolongation, tachycardia, and thrombophlebitis.
Digestive: Infrequent were increased appetite, tongue edema, esophagitis, gastroenteritis, liver function abnormality, and thirst. Rare were anorexia, constipation, gastritis, hematemesis, pancreatitis, melena, and ulcer.
Hemic: Infrequent was ecchymosis. Rare were cyanosis, thrombocytopenia, eosinophilia, and leukopenia.
Metabolic: Infrequent was edema. Rare were hyperglycemia and alkaline phosphatase increased.
Musculoskeletal: Infrequent were back pain, leg cramps, and tenosynovitis. Rare were arthritis, asthenia, tetany, and twitching.
Neurological: Infrequent were agitation, anxiety, depression, emotional lability, and insomnia. Rare were akathisia, amnesia, apathy, ataxia, dystonia, euphoria, hallucinations, cerebral ischemia, hyperkinesia, hypotonia, hypertonia, and irritability.
Respiratory: Infrequent were bronchitis, bronchospasm, epistaxis, hiccup, laryngitis, and yawn. Rare were apnea and voice alteration.
Skin: Infrequent were pruritus, rash, and urticaria.
Special Senses: Infrequent were dry eye, eye pain, hyperacusis, ear pain, parosmia, and tinnitus. Rare were diplopia and lacrimation.
Urogenital: Infrequent were hematuria, cystitis, polyuria, urinary frequency, urinary urgency. Rare were miscarriage and dysmenorrhea.
The adverse experiences profile seen with ZOMIG-ZMT Tablets was similar to that seen with ZOMIG Tablets.
Postmarketing Experience with ZOMIG Tablets:
The following section enumerates potentially important adverse events that have occurred in clinical practice and which have been reported spontaneously to various surveillance systems. The events enumerated represent reports arising from both domestic and non-domestic use of oral zolmitriptan. The events enumerated include all except those already listed in the ADVERSE REACTIONS section above or those too general to be informative. Because the reports cite events reported spontaneously from worldwide postmarketing experience, frequency of events and the role of zolmitriptan in their causation cannot be reliably determined.
Cardiovascular:
Coronary artery vasospasm; transient myocardial ischemia, angina pectoris, and myocardial infarction.
Digestive:
Very rare gastrointestinal ischemic events including splenic infarction, ischemic colitis and gastrointestinal infarction or necrosis have been reported; these may present as bloody diarrhea or abdominal pain. (See WARNINGS.)
Neurological:
As with other acute migraine treatments including other 5HT1 agonists, there have been rare reports of headache.
General:
As with other 5-HT1B/1D agonists, there have been very rare reports of anaphylaxis or anaphylactoid reactions in patients receiving ZOMIG. There have been rare reports of hypersensitivity reactions, including angioedema.
Serotonin syndrome has also been reported during the postmarketing period (see WARNINGS and PRECAUTIONS).
- DRUG ABUSE AND DEPENDENCE:
-
OVERDOSAGE:
There is no experience with clinical overdose. Volunteers receiving single 50 mg oral doses of zolmitriptan commonly experienced sedation.
The elimination half-life of ZOMIG is 3 hours (see CLINICAL PHARMACOLOGY), and therefore monitoring of patients after overdose with ZOMIG should continue for at least 15 hours or while symptoms or signs persist.
There is no specific antidote to zolmitriptan. In cases of severe intoxication, intensive care procedures are recommended, including establishing and maintaining a patent airway, ensuring adequate oxygenation and ventilation, and monitoring and support of the cardiovascular system.
It is unknown what effect hemodialysis or peritoneal dialysis has on the plasma concentrations of zolmitriptan.
-
DOSAGE AND ADMINISTRATION:
ZOMIG Tablets
In controlled clinical trials, single doses of 1, 2.5 and 5 mg of ZOMIG Tablets were effective for the acute treatment of migraines in adults. A greater proportion of patients had headache response following a 2.5 or 5 mg dose than following a 1 mg dose (see Table 1). In the only direct comparison of 2.5 and 5 mg, there was little added benefit from the larger dose but side effects are generally increased at 5 mg (see Table 2). Patients should, therefore, be started on 2.5 mg or lower. A dose lower than 2.5 mg can be achieved by manually breaking the scored 2.5 mg tablet in half.
If the headache returns, the dose may be repeated after 2 hours, not to exceed 10 mg within a 24-hour period. Controlled trials have not adequately established the effectiveness of a second dose if the initial dose is ineffective.
The safety of treating an average of more than three headaches in a 30-day period has not been established.
ZOMIG-ZMT Orally Disintegrating Tablets
In a controlled clinical trial, a single dose of 2.5 mg of ZOMIG-ZMT Tablets was effective for the acute treatment of migraines in adults.
If the headache returns, the dose may be repeated after 2 hours, not to exceed 10 mg within a 24-hour period. Controlled trials have not adequately established the effectiveness of a second dose if the initial dose is ineffective.
The safety of treating an average of more than three headaches in a 30-day period has not been established.
Administration with liquid is not necessary. The orally disintegrating tablet is packaged in a blister. Patients should be instructed not to remove the tablet from the blister until just prior to dosing. The blister pack should then be peeled open, and the orally disintegrating tablet placed on the tongue, where it will dissolve and be swallowed with the saliva. It is not recommended to break the orally disintegrating tablet.
Hepatic Impairment:
Patients with moderate to severe hepatic impairment have decreased clearance of zolmitriptan and significant elevation in blood pressure was observed in some patients. Use of a low dose with blood pressure monitoring is recommended (see CLINICAL PHARMACOLOGY AND WARNINGS).
-
HOW SUPPLIED:
2.5 mg Tablets - Yellow, biconvex, round film-coated, scored tablets containing 2.5 mg of zolmitriptan identified with “ZOMIG” and “2.5” debossed on one side are supplied in cartons containing a blister pack of 6 tablets (NDC 0310-0210-20).
2.5 mg Orally Disintegrating Tablets - White, flat faced, uncoated, bevelled tablet containing 2.5 mg of zolmitriptan identified with a debossed “Z” on one side are supplied in cartons containing a blister pack of 6 tablets (NDC 0310-0209-20).
5 mg Tablets − Pink, biconvex, film-coated tablets containing 5 mg of zolmitriptan identified with “ZOMIG” and “5” debossed on one side are supplied in cartons containing a blister pack of 3 tablets (NDC 0310-0211-25).
5 mg Orally Disintegrating Tablets - White, flat faced, round, uncoated, bevelled tablet containing 5.0 mg of zolmitriptan identified with a debossed “Z” and “5” on one side and plain on the other are supplied in cartons containing a blister pack of 3 tablets (NDC 0310-0213-21).
Store both ZOMIG Tablets and ZOMIG-ZMT Tablets at controlled room temperature, 20-25°C (68-77°F) [see USP]. Protect from light and moisture.
-
PATIENT INFORMATION
The following wording is contained in a separate leaflet provided for patients.
Information for the Consumer on ZOMIG (zolmitriptan) Tablets:
Please read this leaflet carefully before you take ZOMIG Tablets. This provides a summary of the information available on your medicine. Please do not throw away this leaflet until you have finished your medicine. You may need to read this leaflet again. This leaflet does not contain all the information on ZOMIG Tablets. For further information or advice, ask your doctor or pharmacist.
Information About Your Medicine:
The name of your medicine is ZOMIG Tablets. It can be obtained only by prescription from your doctor. The decision to use ZOMIG Tablets is one that you and your doctor should make jointly, taking into account your individual preferences and medical circumstances. If you have risk factors for heart disease (such as high blood pressure, high cholesterol, obesity, diabetes, smoking, strong family history of heart disease, or you are postmenopausal or a male over the age of 40), you should tell your doctor, who should evaluate you for heart disease in order to determine if ZOMIG Tablets are appropriate for you. This medicine was prescribed for you to treat your particular condition and should not be used by others or for any other condition.
-
The Purpose of Your Medicine: ZOMIG Tablets are intended to relieve your migraine, but not to prevent or reduce the number of attacks you experience. Use ZOMIG Tablets only to treat an actual migraine attack.
-
Important Questions to Consider Before Taking ZOMIG Tablets: If the answer to any of the following questions is YES or if you do not know the answer, then you must discuss it with your doctor before you use ZOMIG Tablets.
- Do you have any chest pain, heart disease, shortness of breath, or irregular heartbeats? Have you had a heart attack?
-
Do you have risk factors for heart disease (such as high blood pressure, high cholesterol, obesity, diabetes, smoking, strong family history of heart disease, or you are postmenopausal or a male over the age of 40)?
-
Have you had a stroke or problems with your blood circulation?
-
Do you have high blood pressure?
-
Are you pregnant? Do you think you might be pregnant? Are you trying to become pregnant? Are you not using adequate contraception? Are you breast feeding an infant?
-
If you are taking ZOMIG-ZMT®, are you sensitive to phenylalanine (a component of the artificial sweetener aspartame)?
-
Have you ever had to stop taking this or any other medication because of an allergy or other problems?
-
Are you taking any other migraine medications, including 5-HT1 agonists (triptans) or migraine medications containing ergotamine, dihydroergotamine, or methysergide?
-
Are you taking selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs), two types of drugs for depression or other disorders? Common SSRIs are CELEXA® (citalopram HBr), LEXAPRO® (escitalopram oxalate), PAXIL® (paroxetine), PROZAC® (fluoxetine), SYMBYAX® (olanzapine/fluoxetine), ZOLOFT® (sertraline), SARAFEM® (fluoxetine), and LUVOX® (fluvoxamine). Common SNRIs are CYMBALTA® (duloxetine) and EFFEXOR® (venlafaxine).
-
Are you taking cimetidine for gastrointestinal symptoms?
-
Have you had, or do you have, any disease of the liver or kidney?
-
Have you had, or do you have, epilepsy or seizures?
-
Is this headache different from your usual migraine attacks?
-
The Use of ZOMIG Tablets During Pregnancy: Do not use ZOMIG Tablets if you are pregnant, think you might be pregnant, are trying to become pregnant, or are not using adequate contraception, unless you have discussed this with your doctor.
-
How to Use ZOMIG Tablets and ZOMIG-ZMT Orally Disintegrating Tablets: Adults should be started on a 2.5 mg dose or lower administered by mouth. A dose lower than 2.5 mg can be achieved by manually breaking the conventional film-coated, scored 2.5 mg tablet in half. It is not recommended to break the ZOMIG-ZMT Tablet. If your headache comes back after your initial dose, a second dose may be administered anytime after 2 hours of taking the dose. For any attack where you have no response to the first dose, do not take a second dose without first consulting with your doctor. Do not take more than a total of 10 mg of ZOMIG in any 24-hour period. Discard any unused tablets or its portion that have been removed from the blister packaging. Do not take ZOMIG with any other drug in the same class (triptans) within 24 hours or within 24 hours of taking ergotamine-type medications such as ergotamine, dihydroergotamine or methysergide to treat your migraine.
Additionally for ZOMIG-ZMT Tablets, the blister pack should be peeled open and the orally disintegrating tablet placed on the tongue, where it will dissolve and be swallowed with the saliva. -
Side Effects to Watch for:
-
Some patients experience pain or tightness in the chest or throat, including muscle aches and pains, when using ZOMIG. If this happens to you, then discuss it with your doctor before using any more ZOMIG. If the chest pain is severe or does not go away, call your doctor immediately. As with other drugs in this class (triptans), there have been very rare reports of heart attack occurring in patients with and without risk factors for heart and blood vessel disease.
-
Some people experience: alterations of heart rate; temporary increase in blood pressure; sudden and severe stomach pain. Call your doctor immediately if you have any of these symptoms after taking ZOMIG.
-
Shortness of breath; wheeziness; heart throbbing; swelling of eyelids, face, or lips; or a skin rash, skin lumps, or hives happens rarely. If it happens to you, then tell your doctor immediately. Do not take any more ZOMIG unless your doctor tells you to do so.
-
Some people may have feelings of dry mouth, tingling, heat, heaviness, or pressure after treatment with ZOMIG. A few people may feel drowsy, dizzy, tired, or sick. Tell your doctor immediately if you have symptoms that you do not understand.
-
Some people may have a reaction called serotonin syndrome, which can be life-threatening, when they use ZOMIG. In particular, this reaction may occur when they use ZOMIG together with certain types of antidepressants known as SSRIs or SNRIs. Symptoms may include confusion, hallucinations, fast heart beat, feeling faint, fever, sweating, muscle spasm, difficulty walking, and/or diarrhea. Call your doctor immediately if you have any of these symptoms after taking ZOMIG.
-
-
What To Do If An Overdose Is Taken: If you have taken more medication than you have been told, contact either your doctor, hospital emergency department, or nearest poison control center immediately.
-
Storing Your Medicine: Keep your medicine in a safe place where children cannot reach it. It may be harmful to children. Store your medication away from light and moisture, and at a controlled room temperature. If your medication has expired (the expiration date is printed on the treatment pack), throw it away as instructed. If your doctor decides to stop your treatment, do not keep any leftover medicine unless your doctor tells you to. Throw away your medicine as instructed. Be sure that discarded tablets are out of the reach of children.
ZOMIG and ZOMIG-ZMT are registered trademarks of the AstraZeneca group of companies.
Other brands mentioned are trademarks of their respective owners and are not trademarks of the AstraZeneca group of companies. The makers of these brands are not affiliated with AstraZeneca or its products.
© AstraZeneca 2007, 2008
ZOMIG®(zolmitriptan) Tablets
Manufactured for:
AstraZeneca Pharmaceuticals LP
Wilmington, Delaware 19850
By: IPR Pharmaceuticals, Inc.
Canóvanas, PR 00729
ZOMIG-ZMT® (zolmitriptan) Orally Disintegrating Tablets
Manufactured for:
AstraZeneca Pharmaceuticals LP
Wilmington, Delaware 19850
By: CIMA Labs, Inc.
Eden Prairie, Minnesota 55344
Rev 10/08 SIC 30086–05
-
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZOMIG
zolmitriptan tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68258-7103(NDC:0310-0211) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZOLMITRIPTAN (UNII: 2FS66TH3YW) (ZOLMITRIPTAN - UNII:2FS66TH3YW) ZOLMITRIPTAN 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color pink Score no score Shape ROUND (biconvex; filmcoated) Size 9mm Flavor Imprint Code ZOMIG;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68258-7103-3 3 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020768 05/18/2010 Labeler - Dispensing Solutions, Inc. (066070785) Establishment Name Address ID/FEI Business Operations Dispensing Solutions, Inc. 066070785 relabel, repack


