HEALTHY ACCENTS CORTISONE MAXIMUM STRENGTH- hydrocortisone cream
DZA Brands LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DZA Brands, LLC Cortisone Cream Drug Facts
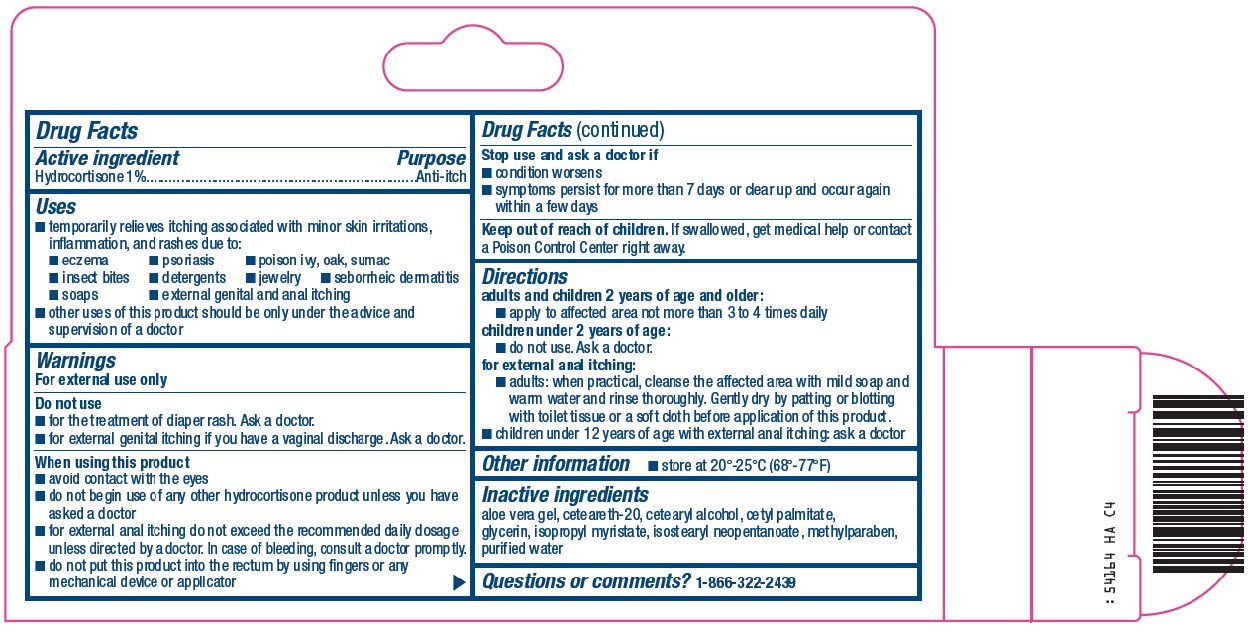
Uses
- •
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
- •
- eczema
- •
- psoriasis
- •
- poison ivy, oak, sumac
- •
- insect bites
- •
- detergents
- •
- jewelry
- •
- seborrheic dermatitis
- •
- soaps
- •
- external genital and anal itching
- •
- other uses of this product should be only under the advice and supervision of a doctor
Warnings
For external use only
Do not use
- •
- for the treatment of diaper rash. Ask a doctor.
- •
- for external genital itching if you have a vaginal discharge. Ask a doctor.
When using this product
- •
- avoid contact with the eyes
- •
- do not begin use of any other hydrocortisone product unless you have asked a doctor
- •
- for external anal itching do not exceed the recommended daily dosage unless directed by a doctor. In case of bleeding, consult a doctor promptly.
- •
- do not put this product into the rectum by using fingers or any mechanical device or applicator
Directions
adults and children 2 years of age and older:
- •
- apply to affected area not more than 3 to 4 times daily
children under 2 years of age:
- •
- do not use. Ask a doctor.
for external anal itching:
- •
- adults: when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
children under 12 years of age with external anal itching: ask a doctor
| HEALTHY ACCENTS CORTISONE
MAXIMUM STRENGTH
hydrocortisone cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - DZA Brands LLC (090322194) |
Revised: 12/2019
Document Id: a2b2448d-6e49-4e03-8e75-c1d600814a17
Set id: 5692eb8b-d09a-48b8-8813-83fe919b8878
Version: 3
Effective Time: 20191211
DZA Brands LLC