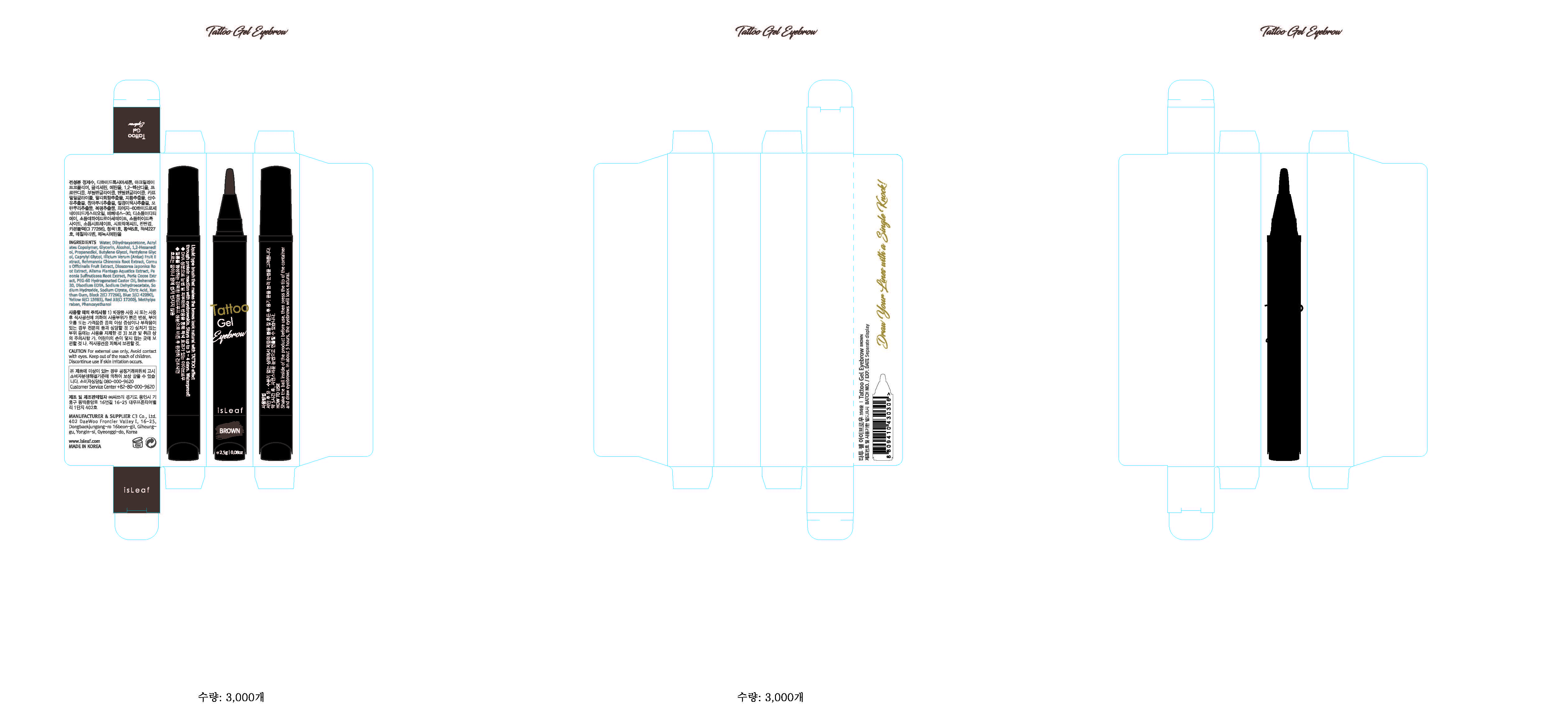
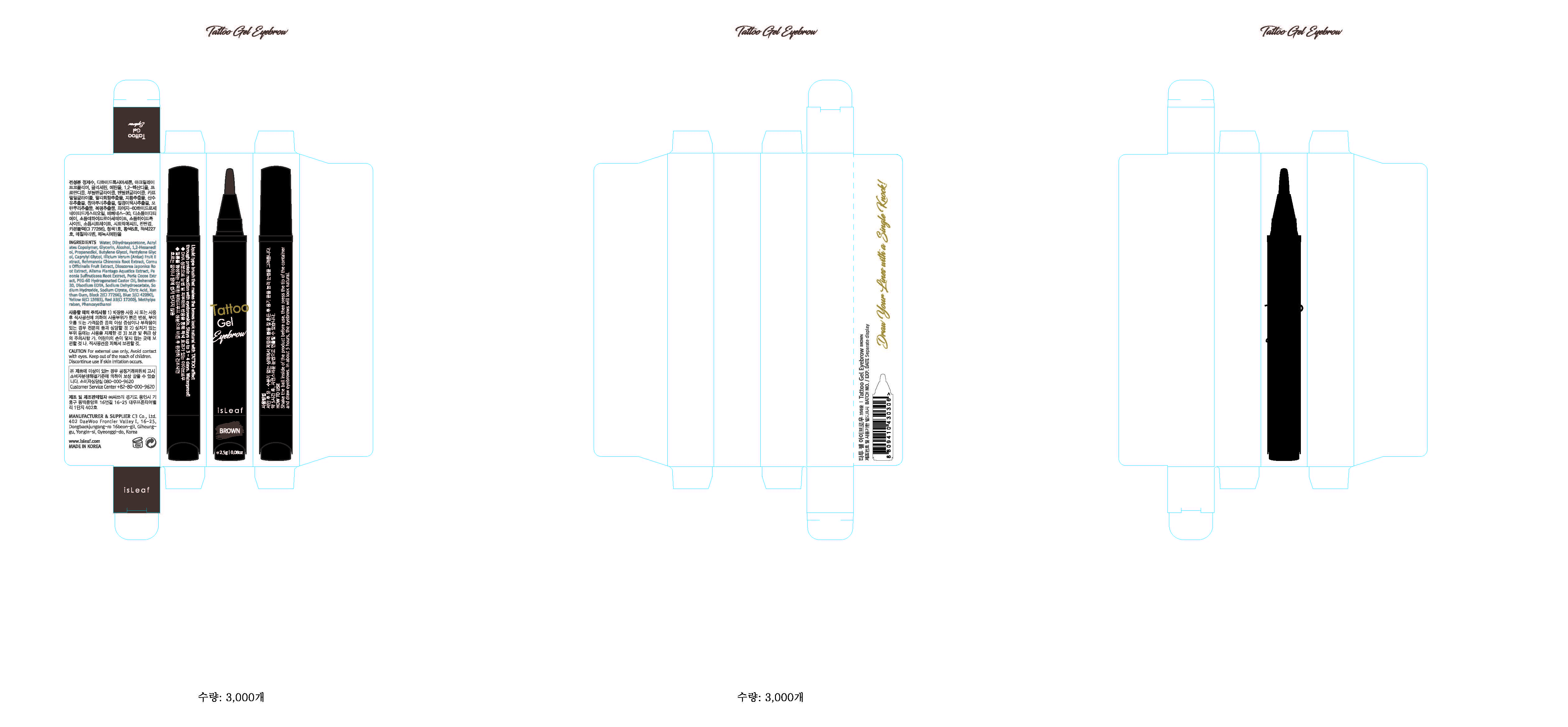
Label: ISLEAF TATTOO EYEBROW BROWN- methylparaben gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 70818-007-01 - Packager: C3 Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 8, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
1. If the following symptoms occur after product use, stop using the product immediately and consult a dermatologist (continuous use can exacerbate the symptoms).
1) Occurrence of red spots, swelling, itchiness, and other skin irritation
2) If the symptoms above occur after the application area is exposed to direct sunlight
2. Do not use on open wounds, eczema, and other skin irritations
3. Precaution for Storage and Handling
1) Close the lid after use
2) Keep out of reach of infants and children
3) Do not to store in a place with high/low temperature and exposed to direct sunlight4. Use as avoiding eye areas.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ISLEAF TATTOO EYEBROW BROWN
methylparaben gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70818-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLPARABEN (UNII: A2I8C7HI9T) (METHYLPARABEN - UNII:A2I8C7HI9T) METHYLPARABEN 0.3 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70818-007-01 2.5 g in 1 APPLICATOR; Type 0: Not a Combination Product 08/08/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/08/2017 Labeler - C3 Co., Ltd. (689846633) Registrant - C3 Co., Ltd. (689846633) Establishment Name Address ID/FEI Business Operations C3 Co., Ltd. 689846633 label(70818-007) , pack(70818-007) , manufacture(70818-007)