Label: PRAZIQUANTEL injection, solution
- NDC Code(s): 61133-1400-1, 61133-1400-2
- Packager: Bimeda, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated January 17, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION:
- INDICATIONS:
-
ACTION:
Praziquantel Injection is absorbed, metabolized in the liver and excreted via the bile into the digestive tract where its cestocidal activity is exerted1. Following exposure to praziquantel, the tapeworm loses its ability to resist digestion by the mammalian host. Because of this, whole tapeworms, including the scolex, are very rarely passed after administration of praziquantel. It is common to see only disintegrated and partially digested pieces of tapeworms in the stool. The majority of tapeworms killed are digested and are not found in the feces.
-
USE DIRECTIONS:
Praziquantel Injection Cestocide may be administered by either the subcutaneous or intramuscular route to dogs and cats. The recommended dosage of praziquantel varies according to body weight. Smaller animals require a relatively larger dosage. The optimum dosage for each individual animal will be achieved by utilizing the following dosage schedule.
DOGS AND PUPPIES† † Not intended for use in puppies less than four (4) weeks of age. Dogs:
5 lbs. and under
0.3 mL
6-10 lbs.
0.5 mL
11-25 lbs.
1.0 mL
over 25 lbs.
0.2 mL/5 lbs.
body weight to a
maximum of 3 mLCATS AND KITTENS†† †† Not intended for use in kittens less than six (6) weeks of age. Cats:
Under 5 lbs.
0.2 mL
5-10 lbs.
0.4 mL
11 lbs. and over
0.6 mL maximum
10 mL: Use within 6 months of first puncture and puncture a maximum of 25 times. Any product remaining after 25 punctures or more than 6 months after initial puncture should be discarded.
50 mL: Use within 6 months of first puncture and puncture a maximum of 90 times. Any product remaining after 90 punctures or more than 6 months after initial puncture should be discarded.
- FASTING:
-
ADMINISTRATION:
Praziquantel Injection Cestocide may be administered by either the subcutaneous or intramuscular route to dogs and cats. The intramuscular route may be preferred in dogs due to a brief period of pain that occasionally follows subcutaneous administration.
Anaphylactoid reactions were not observed in clinical trials. However, as with any drug, an anaphylactoid reaction can occur with this product and should be treated symptomatically if it occurs.
-
RETREATMENT:
For those animals living where reinfections are likely to occur, clients should be instructed in the steps to optimize prevention, otherwise, retreatment may be necessary. This is true in cases of Dipylidium caninum where reinfection is almost certain to occur if fleas are not removed from the animal and its environment. In addition, for control of Echinococcus multilocularis, a program of regular treatment every 21 to 26 days may be indicated (see E. multilocularis section below).
-
ECHINOCOCCUS MULTILOCULARIS:
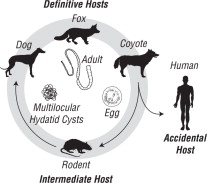
Echinococcus multilocularis is a tapeworm species ordinarily considered to be found in wild canids, including foxes, coyotes and wolves. The parasite has also been identified in domestic dogs and cats and potentially is a serious public health concern by involving humans as accidental intermediate hosts.
The life cycle of the parasite is based on a predator-prey relationship, as depicted above.
The adult tapeworm is small (1-4 mm) and resides in the intestinal tract of the definitive host (wild or domestic canids). Eggs from the adult tapeworm are shed in the feces of the infected canid. Rodents such as mice and voles serve as the intermediate host for E. multilocularis. Eggs ingested by rodents develop in the liver, lungs and other organs to form multilocular cysts. The life cycle is completed after a canid consumes a rodent infected with cysts. After ingestion of the infected rodent, larvae within the cyst develop to adult tapeworms in the intestinal tract of the canid. Eggs may begin to be passed in the feces of the canid approximately 28 days later.
This parasite poses a serious public health problem because of the possibility for human involvement in the life cycle. If eggs shed by an infected canid are accidentally ingested, a highly pathogenic condition (Alveolar Hydatid Disease) results from development of the cyst stage in humans.
The original geographic distribution of E. multilocularis was primarily confined to northern areas of North America. Current evidence indicates migration of the parasite well into the continental United States.2,3
Domestic dogs living in E. multilocularis endemic areas that roam freely with the opportunity to catch wild rodents are at risk for infection. Pet owners should be advised on how to minimize this risk. Proper restraint of roaming dogs should be encouraged, along with regular treatment with Praziquantel Injection solution, following the dosing schedule aforementioned and precautions indicated below.
Additional information on the life cycle and epidemiology of this parasite is available in veterinary parasitology texts.4,5
Diagnosis:
Diagnosis of E. multilocularis in canids is difficult. The adult tapeworm produces no clinical signs of infection. Tapeworm segments (proglottids) are usually not observed in the feces. E. multilocularis eggs, observed using microscopic fecal examination procedures, are similar in appearance to the common taeniid species of canids such as Taenia pisiformis.
Assistance in the diagnosis of E multilocularis may be available from a state veterinary diagnostic laboratory. Additional information regarding areas where E. multilocularis is suspected or has been confirmed may be obtained from area veterinary schools or the Centers for Disease Control in Atlanta, GA.
Treatment:
Dogs infected with E. multilocularis should be treated to prevent exposure of humans to infective eggs and to reduce perpetuation of the parasite's life cycle.
The dosage of Praziquantel Injection solution for removal of E. multilocularis is the same as that indicated for the removal of the other tapeworm species listed on the label. Laboratory efficacy studies have demonstrated the recommended dosage is 100% efficacious for removal of this tapeworm.
Under condition of continual exposure to wild rodents, retreatment of the dog at 21-26 day intervals is recommended to prevent the shedding of infectious eggs.
Precautions:
Strict hygienic precautions should be taken when handling dogs or feces suspected of harboring E. multilocularis. Infected dogs treated for the first time with Praziquantel Injection solution and dogs treated at intervals greater than 28 days may shed eggs in the feces after treatment. The animal should be held in the clinic during this interval and all feces should be incinerated or autoclaved. If these procedures are not possible, the eggs can be destroyed by soaking the feces in a sodium hypochlorite (bleach) solution of 3.75% or greater.6 All areas where the animal was maintained or in contact with should be thoroughly cleaned with sodium hypochlorite and allowed to dry completely before reuse.
-
OVERDOSAGE:
The safety index has been derived from controlled safety evaluations, clinical trials and prior approved use in foreign countries. Dosages of 5 times the labeled rate at 14 day intervals to dogs as young as 4 weeks did not produce signs of clinical toxicity following either intramuscular or subcutaneous injections. No significant clinical chemistry, hematological, cholinesterase or histopathological changes occurred. Dosages of 5 times the labeled rate at 14 day intervals to kittens as young as 5 1/2 weeks did not produce signs of clinical toxicity following either intramuscular or subcutaneous injections. Symptoms of overdosage (33.8 to 40 times the labeled dosage rate) in adult dogs included vomition, excessive salivation and depression, but no deaths. Symptoms of overdosage (10 to 20 times the labeled dosage rate) in adult cats included vomition, depression, muscle tremors and incoordination. Deaths occurred in 5 of 8 cats treated subcutaneously and in all 8 injected intramuscularly at doses greater than 20 times the label rate.
- CONTRAINDICATIONS:
- PREGNANCY:
-
ADVERSE REACTIONS:
Mild side effects were observed in 18 of 189 dogs (9.5%) and 8 of 85 cats (9.4%) administered Praziquantel Injection in field trials. For dogs the majority of these were described as brief pain responses following injections to larger dogs (weighing over 50 lbs.) Two dogs exhibited a brief period of mild vomiting and/or drowsy or staggering gait. The eight cats exhibited either diarrhea, weakness, vomition, salivation, sleepiness, burning on injection and/or a temporary lack of appetite. Local irritation or swelling at the site of subcutaneous injections have been reported for cats.
CAUTION:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
- STORAGE CONDITIONS:
- HOW SUPPLIED:
-
REFERENCES:
- 1.
- Andrews P. Pharmacokinetic Studies with Praziquantel Injection in Animals Using a Biological Assay. Veterinary Medical Review, 2/76, pg. 154-165.
- 2.
- Hildreth MB, Johnson MD and Kazacos KR. 1991. A Zoonosis of Increasing Concern in the United States. Compendium for Cont Ed. 13(5) 727-740.
- 3.
- Lieby PD, Carney WP and Woods CE. 1970. Studies on Sylvatic Echinococcosis, III. Host Occurrence and Geographic Distribution of Echinococcus multilocularis in the North Central United States. J Parasit 56 (6) 1141-1150.
- 4.
- Georgi JR and Georgi ME. 1990. Parasitology for Veterinarians. W.B. Saunders Co. 118-138.
- 5.
- Soulsby EJL. 1982. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th Edition. Lea & Febigir. 118-138.
- 6.
- Craig PS and McPharson CNL. 1998. Sodium Hypochlorite as an Ovicide for Echinococcus. Ann Trop Med and Parasit 82(2) 211 -213.
For customer service or to obtain product information, including Safety Data Sheet, call 1-888-524-6332. For medical emergencies or to report adverse reactions, call 1-888-524-6332.
March, 2020
87186776 LV2003Approved by FDA under NADA #111-607
Manufactured for:
Bimeda, Inc.
Le Sueur, MN 56058
www.bimeda.com
Made in China
Bimeda® -
Principal Display Panel – 10 mL Carton Label
Bimeda®
Praziquantel
(praziquantel) InjectionINJECTABLE CESTOCIDE FOR DOGS AND CATS
56.8 mg/mL SolutionFor Removal Of D. caninum, T. pisiformis,
E. granulosus And For Removal and Control
Of E. multilocularis From Dogs.For Removal Of D. caninum And
T. taeniaeformis From Cats.Approved by FDA under NADA # 111-607
10 mL
- Principal Display Panel – 10 mL Vial Label
-
INGREDIENTS AND APPEARANCE
PRAZIQUANTEL
praziquantel injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:61133-1400 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 56.8 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-1400-1 1 in 1 CARTON 1 10 mL in 1 VIAL 2 NDC:61133-1400-2 1 in 1 CARTON 2 50 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA111607 04/01/2013 Labeler - Bimeda, Inc. (060492923)