Label: PHENTERMINE HYDROCHLORIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 52959-812-21, 52959-812-45 - Packager: H.J. Harkins Company, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated January 3, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Indications & Usage
Phentermine hydrochloride tablets USP are indicated as a short-term (a few weeks) adjunct in a regimen
of weight reduction based on exercise, behavioral modification and caloric restriction in the
management of exogenous obesity for patients with an initial body mass index greater than or equal to
30 kg/m , or greater than or equal to 27 kg/m in the presence of other risk factors (e.g., controlled
hypertension, diabetes, hyperlipidemia).
Below is a chart of body mass index (BMI) based on various heights and weights.BMI is calculated by taking the patient's weight, in kilograms (kg), divided by the patient's height, in meters (m), squared. Metric conversions are as follows: pounds ÷ 2.2 = kg; inches x 0.0254 = meters.
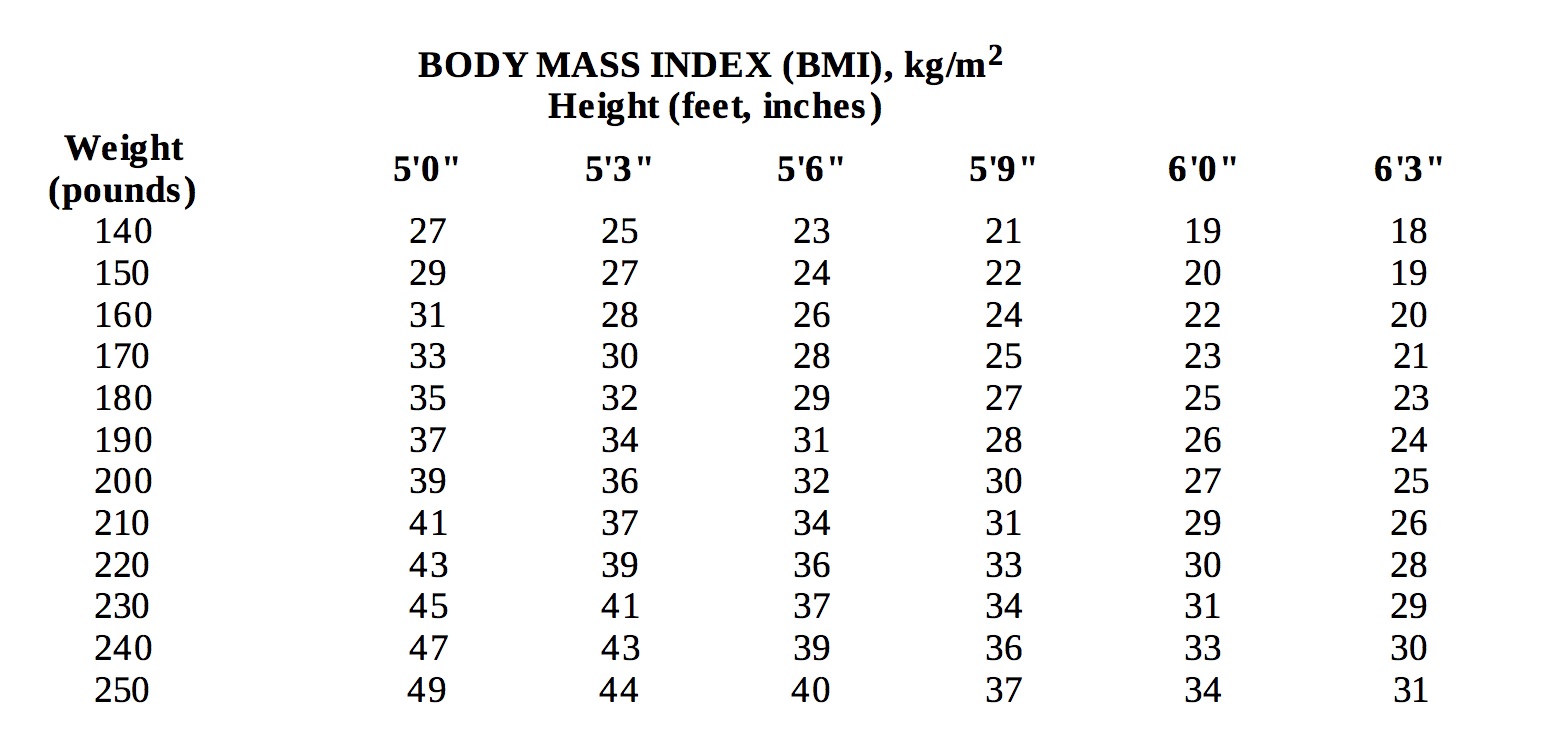
-
Description
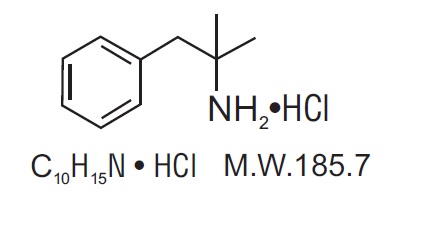
Phentermine hydrochloride USP is a sympathomimetic amine anorectic. Its chemical name is a,a- dimethylphenethylamine hydrochloride. The structural formula is as follows:
Phentermine hydrochloride USP is a white, odorless, hygroscopic, crystalline powder which is soluble in water and lower alcohols, slightly soluble in chloroform and insoluble in ether.
Phentermine hydrochloride tablets USP are available as an oral tablet containing 37.5 mg of phentermine hydrochloride USP (equivalent to 30 mg of phentermine base). Each phentermine hydrochloride tablet USP also contains the inactive ingredients microcrystalline cellulose, pregelatinized starch, anhydrous lactose, crospovidone, colloidal silicon dioxide, magnesium stearate, sucrose, corn starch and FD&C Blue #1.
- Package and Display
-
INGREDIENTS AND APPEARANCE
PHENTERMINE HYDROCHLORIDE
phentermine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52959-812 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENTERMINE HYDROCHLORIDE (UNII: 0K2I505OTV) (PHENTERMINE - UNII:C045TQL4WP) PHENTERMINE HYDROCHLORIDE 37.5 mg Product Characteristics Color white (Off-white with blue specks) Score no score Shape OVAL Size 10mm Flavor Imprint Code MP;273 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52959-812-21 21 in 1 BOTTLE; Type 0: Not a Combination Product 07/18/2017 2 NDC:52959-812-45 45 in 1 CONTAINER; Type 0: Not a Combination Product 07/18/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203068 07/18/2017 Labeler - H.J. Harkins Company, Inc. (147681894) Establishment Name Address ID/FEI Business Operations H.J. Harkins Comapny., Inc. 147681894 repack(52959-812) , relabel(52959-812) , manufacture(52959-812)


