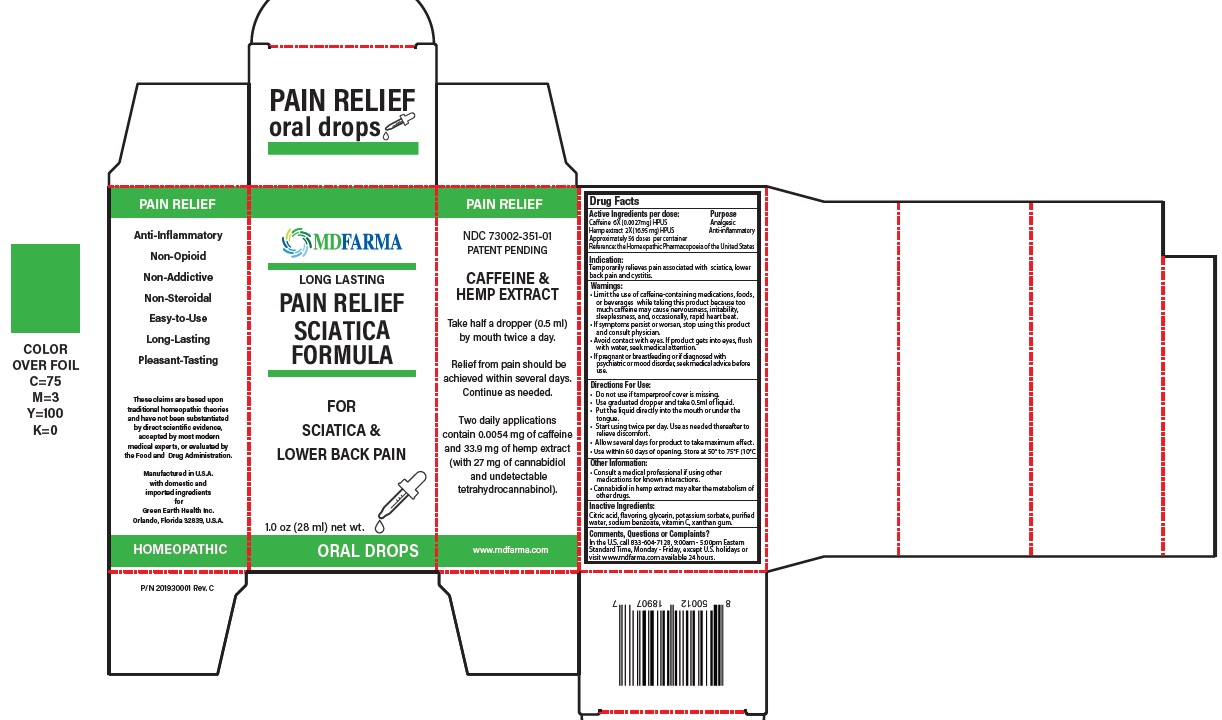
Label: PAIN RELIEF CAFFEINE AND HEMP (caffeine (coffeinum) 6x, hemp extract- cannabis sativa 2x liquid
- NDC Code(s): 73002-351-01
- Packager: Green Earth Health Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 27, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Indication:
- KEEP OUT OF REACH OF CHILDREN
-
Warnings:
- Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
- If symptoms persist or worsen, stop using this product and consult physician.
- Avoid contact with eyes. If product gets into eyes, flush with water, seek medical attention.
- If pregnant or breastfeeding or if diagnosed with psychiatric or mood disorder, seek medical advice before use.
- Keep out of reach of children.
-
Directions For Use:
- Do not use if tamperproof cover is missing.
- Use graduated dropper and take 0.5ml of liquid.
- Put the liquid directly in to the mouth or under the tongue.
- Start using twice per day. Use as needed thereafter to relieve discomfort.
- Allow several days for product to take maximum effect.
- Use within 60 days of opening. Store at 50° to 75°F (10°C to 24°C).
- Other Information:
- Inactive Ingredients:
- Product label
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF CAFFEINE AND HEMP
caffeine (coffeinum) 6x, hemp extract (cannabis sativa) 2x liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73002-351 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE CITRATE (UNII: U26EO4675Q) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE CITRATE 6 [hp_X] in 1 mL CANNABIS SATIVA SUBSP. SATIVA FLOWERING TOP (UNII: 8X454SZ22D) (CANNABIS SATIVA SUBSP. SATIVA FLOWERING TOP - UNII:8X454SZ22D) CANNABIS SATIVA SUBSP. SATIVA FLOWERING TOP 2 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) SIRAITIA GROSVENORII FRUIT (UNII: NOU2FB51TW) TEA LEAF (UNII: GH42T47V24) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) ASCORBIC ACID (UNII: PQ6CK8PD0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73002-351-01 28 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 09/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/01/2020 Labeler - Green Earth Health Inc. (116983264) Registrant - Green Earth Health Inc. (116983264)