Label: VONVENDI (von willebrand factor- recombinant kit
-
NDC Code(s):
0944-7550-01,
0944-7551-02,
0944-7552-01,
0944-7553-02, view more64764-515-50, 64764-516-10
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: PLASMA DERIVATIVE
Drug Label Information
Updated April 7, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VONVENDI safely and effectively. See full prescribing information for VONVENDI.
VONVENDI [von Willebrand factor (recombinant)] lyophilized powder for solution, for intravenous injection
Initial U.S. Approval: 2015INDICATIONS AND USAGE
VONVENDI [von Willebrand factor (recombinant)] is a recombinant von Willebrand factor (rVWF) indicated for use in adults (age 18 and older) diagnosed with von Willebrand disease (VWD) for:
DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
On-Demand Treatment and Control of Bleeding Episodes
- For each bleeding episode, administer the first dose of VONVENDI with an approved recombinant (non-von Willebrand factor containing) factor VIII, if factor VIII baseline levels are below 40% or are unknown. (2.1)
- Initial dose is 40 to 80 International Units (IU) per kg body weight (BW). Adjust the dosage based on the extent and location of bleeding. (2.1)
Bleeding Episode Initial Dose Subsequent Dose Minor 40 to 50 IU/kg 40 to 50 IU/kg every 8 to 24 hours Major 50 to 80 IU/kg 40 to 60 IU/kg every 8 to 24 hours for approximately 2 to 3 days Perioperative Management of Bleeding
For Elective Surgical Procedure
- A dose of VONVENDI may be given 12 to 24 hours prior to surgery to allow the endogenous factor VIII levels to increase to at least 30 IU/dL (minor surgery) or 60 IU/dL (major surgery). (2.1)
- Assess FVIII:C levels within 3 hours prior to surgery. If the FVIII:C levels are at or above the recommended minimum target levels, administer a dose of VONVENDI alone within 1 hour prior to the procedure. If the FVIII:C levels are below the recommended minimum target levels, administer recombinant factor VIII in addition to VONVENDI to raise VWF:RCo and FVIII:C. (2.1)
For Emergency Surgery
- Assess baseline VWF:RCo and FVIII:C levels within 3 hours prior to surgery. If not available, use weight-based dosing calculation. (2.1)
- Administer VONVENDI 1 hour before surgery with or without recombinant factor VIII and adjust the dose to raise VWF:RCo and FVIII:C to adequate level. (2.1)
Type of Surgery Target Peak Plasma Level Calculation of rVWF Dose
(IU VWF:RCo required)VWF:RCo FVIII:C Minor 50 to 60 IU/dL 40 to 50 IU/dL ∆ VWF:RCo × BW (kg)/IR Major 100 IU/dL 80 to 100 IU/dL ∆ VWF:RCo × BW (kg)/IR - Continue to monitor the VWF:RCo and FVIII:C plasma levels after surgical procedure. (2.1)
Routine Prophylaxis to Reduce the Frequency of Bleeding Episodes in Patients with Severe Type 3 VWD Receiving On-Demand Therapy
Initial dosage of VONVENDI is 40 to 60 IU/kg body weight to be administered twice weekly. The dose may be adjusted up to 60 IU/kg twice weekly based on frequency of bleeding episodes. (2.1)
DOSAGE FORMS AND STRENGTHS
VONVENDI is available as a lyophilized powder in single-dose vials containing nominally 650 or 1300 international units VWF:RCo. (3)
CONTRAINDICATIONS
Do not use in patients who have had life-threatening hypersensitivity reactions to VONVENDI or its components (tri-sodium citrate-dihydrate, glycine, mannitol, trehalose-dihydrate polysorbate 80, and hamster or mouse proteins). (4)
WARNINGS AND PRECAUTIONS
- Thromboembolic reactions can occur, particularly in patients with risk factors for thrombosis. Monitor for early signs of thrombosis and have prophylaxis measures against thromboembolism instituted according to current recommendations. One out of 100 VWD subjects treated with VONVENDI in clinical trials developed proximal deep vein thrombosis in perioperative period after undergoing total hip replacement surgery. In patients requiring frequent doses of VONVENDI in combination with recombinant factor VIII, monitor plasma levels for FVIII:C because sustained excessive factor VIII plasma levels can increase the risk for thromboembolic events. (5.1)
- Hypersensitivity reactions, including anaphylaxis, may occur. Discontinue VONVENDI if hypersensitivity symptoms occur and administer appropriate emergency treatment. (5.2)
- Inhibitors to von Willebrand factor (VWF) and/or factor VIII can occur. If the expected plasma levels of VWF activity (VWF:RCo) are not attained, or if bleeding is not controlled with an appropriate dose, perform an appropriate assay to determine if an anti-VWF or anti-factor VIII inhibitors are present. (5.3)
ADVERSE REACTIONS
- The most common adverse reactions observed (≥2% of subjects) were headache, vomiting, nausea, dizziness, arthralgia, joint injury, vertigo, ALT increased and generalized pruritus. (6.1)
- One subject treated with VONVENDI in perioperative setting developed deep vein thrombosis after undergoing total hip replacement surgery. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embolism and Thrombosis
5.2 Hypersensitivity Reactions
5.3 Neutralizing Antibodies
5.4 Monitoring Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
6.3 Immunogenicity
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VONVENDI [von Willebrand factor (recombinant)] is a recombinant von Willebrand factor (rVWF) indicated for use in adults (age 18 and older) diagnosed with von Willebrand disease (VWD) for:
- On-demand treatment and control of bleeding episodes.
- Perioperative management of bleeding.
- Routine Prophylaxis to reduce the frequency of bleeding episodes in patients with severe Type 3 VWD receiving on-demand therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
For intravenous use after reconstitution only.
- Each vial of VONVENDI is labeled with the actual amount of rVWF activity in International Units (IU), as measured with the Ristocetin cofactor assay (VWF:RCo).
- Individualize dosage and frequency according to clinical judgement and based on the subject's weight, type and severity of the bleeding episodes/surgical intervention and based on monitoring of appropriate clinical and laboratory measures.
- For patients experiencing bleeding, hemostasis cannot be ensured until factor VIII coagulation activity (FVIII:C) has reached 40 IU/deciliter (dL) (i.e., 40% of normal activity). If the patient's baseline plasma FVIII:C level is below 40%, or is unknown, administer an approved recombinant (non-von Willebrand factor containing) factor VIII (rFVIII) with the first infusion of VONVENDI in order to achieve a hemostatic plasma level of FVIII:C. However, if an immediate rise in FVIII:C is not necessary or if the baseline FVIII:C level is sufficient to ensure hemostasis, administer VONVENDI without rFVIII.
- Depending on the patient's baseline FVIII:C level, a single infusion of VONVENDI is expected, in a majority of patients, to lead to an increase in endogenous FVIII:C activity above 40% within 6 hours.
- When repeated infusions are required, monitor factor VIII levels to determine if rFVIII is required with subsequent infusions.
On-Demand Treatment and Control of Bleeding Episodes
Administer initial dose of 40 to 80 IU/kg body weight (BW). Dosing guidelines for treatment of minor and major bleeds are provided in Table 1.
Administer VONVENDI within the designated ranges based on clinical judgment, taking into account severity, site of bleeding, and medical history of the patient. Adjust the dose based on the extent and location of the bleeding episode. Administer subsequent doses as long as clinically required. Monitor appropriate clinical and laboratory measures [see Warnings and Precautions (5.2, 5.3)].
Table 1: Dosing Guidelines for Treatment of Minor and Major Bleeding Episodes Bleeding Episodes Initial Dose* Subsequent Dose
(as clinically required)- *
- If recombinant factor VIII is administered, see recombinant factor VIII package insert for reconstitution and administration instructions.
- †
- A bleed could be considered major if red blood cell transfusion is either required or potentially indicated or if bleeding occurs in a critical anatomical site (e.g., intracranial or gastrointestinal hemorrhage).
Minor (e.g., readily managed epistaxis, oral bleeding, menorrhagia) 40 to 50 IU/kg 40 to 50 IU/kg every 8 to 24 hours Major† (e.g., severe or refractory epistaxis, menorrhagia, GI bleeding, CNS trauma, hemarthrosis, or traumatic hemorrhage) 50 to 80 IU/kg 40 to 60 IU/kg every 8 to 24 hours for approximately 2 to 3 days The initial dose of VONVENDI should achieve greater than 60% of von Willebrand factor (VWF) levels (based on VWF:RCo greater than 60 IU/dL) and an infusion of rFVIII should achieve factor VIII levels greater than 40% (FVIII:C greater than 40 IU/dL). In major bleeding episodes, maintain trough levels of VWF:RCo greater than 50% for as long as deemed necessary.
Administer VONVENDI with rFVIII if the FVIII:C level is less than 40%, or is unknown, to control bleeding. The rFVIII dose should be calculated according to the difference between the patient's baseline plasma FVIII:C level, and the desired peak FVIII:C level to achieve an appropriate plasma FVIII:C level based on the approximate mean recovery of 2 (IU/dL)/(IU/kg). Administer the complete dose of VONVENDI followed by rFVIII within 10 minutes.
Calculating Dose
VONVENDI dose [IU] = dose in [IU/kg] × body weight [kg]
In a major bleeding episode when baseline factor VIII level is unknown, rFVIII should be administered to achieve a target peak level of FVIII:C 80 to100 IU/dL, based on the approximate mean recovery of 2 (IU/dL)/(IU/kg). Refer to label for weight-based dose calculation of rFVIII that is used.
If expected VWF activity plasma levels are not attained, or if bleeding episode is not controlled with an appropriate dose, perform an assay that measures the presence of VWF or factor VIII inhibitors [see Warnings and Precautions (5.3)].
Perioperative Management of Bleeding
Elective Surgical Procedures
A preoperative dose of VONVENDI may be administered 12 to 24 hours prior to surgery to allow the endogenous factor VIII levels to increase to at least 30 IU/dL (minor surgery) or 60 IU/dL (major surgery) before the loading dose (1 hour preoperative dose) of rVWF, with or without rFVIII, is administered.
Ensure baseline FVIII:C level is available prior to determining the need for 12 to 24 hour preoperative dose. FVIII:C level should also be assessed within 3 hours prior to initiating the surgical procedure. If the level is at the recommended minimum target levels (30 IU/dL for minor surgery and 60 IU/dL for major surgery), administer a dose of VONVENDI alone (without factor VIII treatment) within 1 hour prior to the procedure. If the FVIII:C level is below the recommended minimum target level, administer complete dose of VONVENDI followed by rFVIII within 10 minutes to raise VWF:RCo and FVIII:C.
Refer to Table 2 for recommended VWF:RCo and FVIII:C target peak plasma levels and dosing guidelines for perioperative management of bleeding.
Assess baseline VWF:RCo levels within 3 hours of administration of the 12 to 24 hour preoperative dose. If the 12 to 24 hour preoperative dose is not administered, then assess baseline level VWF:RCo prior to surgery.
When possible, measure incremental recovery (IR) for VONVENDI before surgery. For calculation of IR, measure baseline plasma VWF:RCo. Then infuse a dose of 50 IU/kg of VONVENDI. Measure VWF:RCo, 30 minutes after infusion of VONVENDI.
Use the following formula to calculate IR:
IR = [Plasma VWF:RCo at 30 minutes (IU/dL) – Plasma VWF:RCo at baseline (IU/dL)]/Dose (IU/kg)
Emergency Surgery
A 12 to 24 hour preoperative dose may not be feasible in subjects requiring emergency surgery. Baseline VWF:RCo and FVIII:C levels should be assessed within 3 hours prior to initiating the surgical procedure if it is feasible. The loading dose (1 hour preoperative dose) can be calculated as the difference in the target peak and baseline plasma VWF:RCo levels divided by the IR. If the IR is not available, assume an IR of 2.0 IU/dL per IU/kg.
If baseline VWF:RCo and FVIII:C is not available, as a general guidance a loading dose (1 hour preoperative dose) of VONVENDI, 40 to 60 IU/kg VWF:RCo, should be administered. Additionally, rFVIII at a dose of 30 to 45 IU/kg may be infused sequentially, preferably within 10 minutes after the VONVENDI infusion in patients whose factor VIII plasma levels already are (or are highly likely to be) less than 40 to 50 IU/dL for minor surgery or 80 to 100 IU/dL for major surgery.
Refer to Table 2 for recommended VWF:RCo and FVIII:C target peak plasma levels and dosing guidelines for perioperative management of bleeding.
Table 2: Recommended VWF:RCo and FVIII:C Target Peak Plasma Levels for the Perioperative Management of Bleeding Type of Surgery VWF:RCo Target Peak Plasma Level FVIII:C Target Peak Plasma Level* Calculation of rVWF Dose
(to be administered within 1 hour prior to surgery) (IU VWF:RCo required)- *
- Additional recombinant factor VIII may be required to attain the recommended FVIII:C target peak plasma levels. Dosing guidance should be done based on the IR. If the IR is not available, assume an IR of 2.0 IU/dL per IU/kg.
Calculation of factor VIII dose: Target FVIII:C - Baseline FVIII:C/IR - †
- ∆ = Target peak plasma VWF:RCo – **baseline plasma VWF:RCo
** Baseline plasma VWF:RCo is VWF:RCo activity level assessed within 3 hours prior to administration of 12 to 24 hours preoperative dose of VONVENDI. If no dose is administered 12 to 24 hours preoperatively, it's recommended to use the VWF:RCo levels prior to surgery. - ‡
- IR = Incremental Recovery as measured in the subject. If the IR is not available, assume an IR of 2.0 IU/dL per IU/kg.
Minor 50 to 60 IU/dL 40 to 50 IU/dL ∆† VWF:RCo × BW (kg) /IR‡ Major 100 IU/dL 80 to 100 IU/dL ∆† VWF:RCo × BW (kg) /IR‡ In the absence of available baseline FVIII:C, VWF:RCo and Incremental Recovery, it is recommended to use body weight-based dosing as outlined below in Table 3.
Table 3: Recommended Body Weight (BW) Based Dosing for the Perioperative Management of Bleeding Type of Surgery VWF:RCo (IU VWF:RCo/kg BW) VWF:RCo Target Peak Plasma Level FVIII:C (IU FVIII:C/kg BW) FVIII:C Target Peak Plasma Level Minor 25 to 30 IU/kg 50 to 60 IU/dL 20 to 25 IU/kg 40 to 50 IU/dL Major 50 ± 10 IU/kg 100 IU/dL 40 to 50 IU/kg 80 to 100 IU/dL Monitor VWF:RCo and FVIII:C plasma levels starting 12 to 24 hours after surgery and at least every 24 hours in the perioperative period to adjust the dosing of VONVENDI or rFVIII levels. VWF:RCo and FVIII:C plasma levels should be monitored and the intra- and postoperative maintenance regimen should be individualized according to the pharmacokinetic (PK) results and intensity and duration of the hemostatic challenge. The frequency of VONVENDI dosing should range between twice a day and every 48 hours.
Refer to Table 4 for recommended VWF:RCo and FVIII:C target trough plasma levels and minimum duration of treatment for subsequent maintenance doses after surgery.
Table 4: Recommended VWF:RCo and FVIII:C Target Trough Plasma Levels and Minimum Duration of Treatment for Subsequent Maintenance Doses Type of Surgery VWF:RCo
Target Trough Plasma LevelFVIII:C
Target Trough Plasma LevelMinimum Duration of Treatment Frequency of Dosing up to 72 Hours Post-Surgery after 72 Hours Post-Surgery up to 72 Hours Post-Surgery after 72 Hours Post-Surgery Minor ≥30 IU/dL - >30 IU/dL - 48 hours Every 12 to 24 hours to every other day Major >50 IU/dL >30 IU/dL >50 IU/dL >30 IU/dL 72 hours Routine Prophylaxis to Reduce the Frequency of Bleeding Episodes in Patients with Severe Type 3 VWD Receiving On-Demand Therapy
For initiation of prophylactic treatment, administer 40 to 60 IU of VONVENDI per kg of body weight twice weekly. Adjust prophylaxis dose up to 60 IU/kg twice weekly if breakthrough bleeding occurs in joints or if severe bleeding occurs. Treat breakthrough bleeding as per the dosing guidelines in Table 1.
2.2 Preparation and Reconstitution
- Allow VONVENDI and Sterile Water for Injection (diluent) to reach room temperature.
- If the patient requires more than one vial of VONVENDI per injection, reconstitute each vial according to the following instructions.
- Some flakes or particles may remain in the reconstituted vial. The filter included in the Mix2Vial device will remove extraneous flakes or particles, and the resulting solution in the syringe should be clear and colorless. Do not use the solution in the syringe if it is cloudy or contains flakes or particles after filtration from the vial into the syringe.
Reconstitution
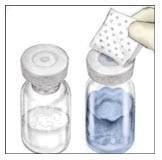
- Remove the plastic caps from the VONVENDI and water for injection vials.
- Clean the rubber stoppers with a sterile alcohol swab.
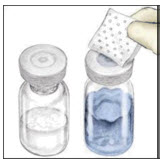
- Peel back the cover of the Mix2Vial transfer device. To maintain sterility, leave the Mix2Vial device in the clear plastic packaging.
- While firmly holding the diluent vial on a level surface, take the Mix2Vial in its plastic package and invert it over the diluent vial. Push the blue plastic cannula of the Mix2Vial firmly straight down through the rubber stopper. Carefully remove the plastic package leaving the Mix2Vial attached firmly to the diluent vial.
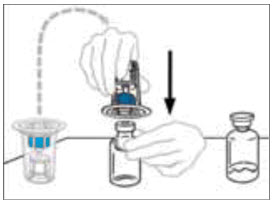
- Hold the VONVENDI vial firmly on a level surface, quickly invert the diluent vial with the Mix2Vial attached and push the transparent plastic cannula end of the Mix2Vial firmly straight down through the stopper of the VONVENDI vial. The diluent will be drawn into the VONVENDI vial by the vacuum.
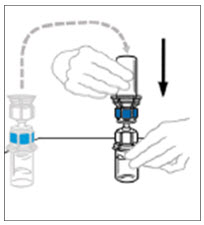
- Verify that diluent transfer is complete. Do not use if vacuum has been lost.
- With both vials still attached, gently swirl the vials or allow the reconstituted product to sit for 5 minutes then gently swirl to ensure the powder is completely dissolved.
Do not shake. Shaking will adversely affect the integrity of the product.
Note: Some flakes or particles may remain in the reconstituted vial. The filter included in the Mix2Vial device will remove extraneous flakes or particles, and the resulting solution in the syringe should be clear and colorless. Do not use the solution in the syringe if it is cloudy or contains flakes or particles after filtration from the vial into the syringe.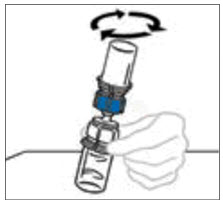
- Once the content is completely dissolved, firmly hold both the transparent and blue parts of the Mix2Vial. Unscrew the Mix2Vial into two separate pieces and discard the empty diluent vial and the blue part of the Mix2Vial. Note: The Mix2Vial is intended for one-time use with a single vial of VONVENDI and diluent only. If the dose requires more than one vial of VONVENDI, reconstitute each vial separately.
Do not refrigerate after reconstitution.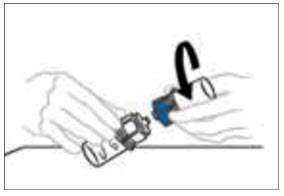
2.3 Administration
For intravenous administration only.
- Administer VONVENDI immediately after reconstitution. If not, store at room temperature not to exceed 25°C (77°F) for up to 3 hours. Discard after 3 hours.
- No more than two vials of VONVENDI may be pooled into a single syringe. Pooling of more than two vials into a syringe may result in formation of filaments, which requires discarding of the solution in the syringe. If a patient is to receive more than one vial of VONVENDI, leave syringe attached to the vial or cover syringe tip with a suitable sterile cap until ready to infuse to reduce risk of contamination.
- Use plastic syringes with this product because proteins in the product tend to stick to the surface of glass syringes.
- Do not mix VONVENDI with other medicinal products.
Administration
- Draw air into an empty, sterile disposable plastic syringe. The amount of air should equal the amount of reconstituted VONVENDI to be withdrawn from the vial.
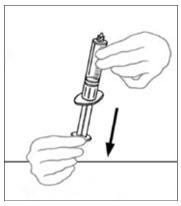
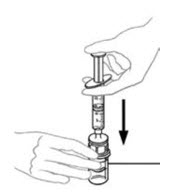
- Leaving the VONVENDI vial (containing the dissolved product) on your flat work surface, connect the syringe to the clear plastic connector by attaching and turning the syringe clockwise (Figure A). Hold the vial with one hand and use the other hand to push the entire amount of air from the syringe into the vial (Figure B). The required amount of product will not be drawn into the syringe if all the air is not pushed into the vial.
Figure A Figure B 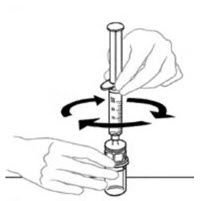
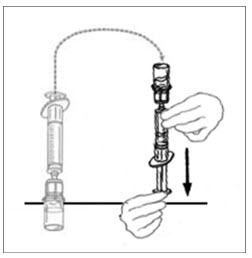
- Flip connected syringe and VONVENDI vial so the vial is on top. Be sure to keep the syringe plunger pressed in. Draw the VONVENDI into the syringe by pulling plunger back slowly. Do not push and pull solution back and forth between syringe and vial. Doing so may harm the integrity of the product.
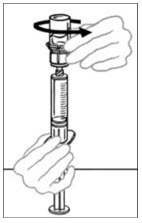
- Inspect VONVENDI after filtration/withdrawal into the syringe for discoloration and particulate matter prior to administration. The solution should be clear or slightly opalescent in appearance. Do not administer if particulate matter, discoloration, or cloudiness is observed and notify Takeda Medical Information 1-877-TAKEDA-7 (1-877-825-3327).
- When ready to infuse, firmly hold the barrel of the syringe (keeping the syringe plunger facing down) and detach the Mix2Vial from the syringe. Discard the Mix2Vial (transparent plastic part) and the empty VONVENDI vial. If a patient is to receive more than one vial of VONVENDI, the contents of up to two vials may be drawn into a single syringe. When pushing air into a second vial of VONVENDI to be pooled into a syringe, position the vial and connected syringe so that the vial is on top.
- Clean the intended injection site with a sterile alcohol swab.
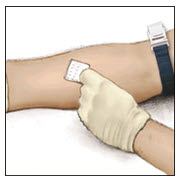
- Attach a suitable infusion needle to the syringe. Infuse intravenously at a rate slow enough that ensures the comfort of the patient, up to a maximum of 4 mL per minute.
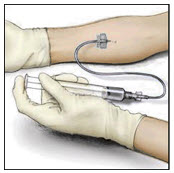
- If tachycardia occurs, the injection speed must be reduced or the administration must be interrupted.
- Dispose of any unused product or waste material in accordance with local requirements.
-
3 DOSAGE FORMS AND STRENGTHS
VONVENDI is available as a non-pyrogenic, white to off-white, lyophilized powder for reconstitution in single-dose vials containing nominally 650 or 1300 IU VWF:RCo/vial.
Each VONVENDI vial is labeled with the number of units of VWF:RCo expressed in IU, which are based on the current World Health Organization (WHO) standard for VWF concentrate.
-
4 CONTRAINDICATIONS
VONVENDI is contraindicated in patients who have had life-threatening hypersensitivity reactions to VONVENDI or constituents of the product (tri-sodium citrate-dihydrate, glycine, mannitol, trehalose-dihydrate, polysorbate 80, and hamster or mouse proteins) [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Embolism and Thrombosis
Thromboembolic reactions, including disseminated intravascular coagulation (DIC), venous thrombosis, pulmonary embolism, myocardial infarction, and stroke, can occur, particularly in patients with known risk factors for thrombosis, including low ADAMTS13 levels [see Description (11)]. Monitor for early signs and symptoms of thrombosis such as pain, swelling, discoloration, dyspnea, cough, hemoptysis, and syncope, and have prophylaxis measures against thromboembolism instituted according to current recommendations and standard of care.
In patients requiring frequent doses of VONVENDI in combination with rFVIII, monitor plasma levels for FVIII:C activity because sustained excessive factor VIII plasma levels can increase the risk of thromboembolic complications.
Of the 100 subjects treated with VONVENDI in clinical trials, one subject was diagnosed with deep vein thrombosis, which was revealed by imaging conducted as a part of the hospital's standard of care for high-risk patients, 3 days after total hip replacement surgery while receiving VONVENDI.
5.2 Hypersensitivity Reactions
Hypersensitivity reactions have occurred with VONVENDI. These reactions can include anaphylactic shock, generalized urticaria, angioedema, chest tightness, hypotension, shock, lethargy, nausea, vomiting, paresthesia, pruritus, restlessness, blurred vision, wheezing and/or acute respiratory distress. If signs and symptoms of severe allergic reactions occur, immediately discontinue administration of VONVENDI and provide appropriate supportive care.
VONVENDI contains trace amounts of mouse immunoglobulin G (MuIgG) and hamster proteins less than or equal to 2 ng/IU VONVENDI. Patients treated with this product may develop hypersensitivity reactions to non-human mammalian proteins.
5.3 Neutralizing Antibodies
Neutralizing antibodies (inhibitors) to VWF and/or factor VIII can occur. If the expected plasma levels of VWF activity (VWF:RCo) are not attained, perform an appropriate assay to determine if anti-VWF or anti-factor VIII inhibitors are present. Consider other therapeutic options and direct the patient to a physician with experience in the care of either VWD or hemophilia A.
In patients with high levels of inhibitors to VWF or factor VIII, VONVENDI therapy may not be effective and infusion of this protein may lead to severe hypersensitivity reactions. Since inhibitor antibodies can occur concomitantly with anaphylactic reactions, evaluate patients experiencing an anaphylactic reaction for the presence of inhibitors.
5.4 Monitoring Laboratory Tests
Monitor plasma levels of VWF:RCo and factor VIII activities in patients receiving VONVENDI to avoid sustained excessive VWF and/or factor VIII activity levels, which may increase the risk of thrombotic events, particularly in patients with known clinical or laboratory risk factors.
Monitor for development of VWF and/or factor VIII inhibitors when suspected. Perform appropriate inhibitor assays to determine if VWF and/or factor VIII inhibitors are present if bleeding is not controlled with the expected dose of VONVENDI.
-
6 ADVERSE REACTIONS
The most common adverse reactions observed in greater than or equal to 2% of subjects in clinical trials with VONVENDI (n=100) were headache, vomiting, nausea, dizziness, arthralgia, joint injury, vertigo, ALT increased and generalized pruritus.
One subject treated with VONVENDI in the surgery study for perioperative management of bleeding developed proximal deep vein thrombosis postoperatively [see Warning and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety profile of VONVENDI was evaluated in five prospective, multicenter trials; four were conducted in subjects with VWD (n=100) and one was conducted in subjects with hemophilia A (n=12). The adverse drug reaction (ADR) terms listed below (Table 5) were assessed by the sponsor/company as having a plausible causal relationship to the study medication in three clinical trials (n=80) during which VONVENDI was used for PK assessment, on-demand treatment and perioperative management of bleeding episodes in patients undergoing surgery.
Table 5: Summary of Adverse Reactions from Completed Clinical Trials in PK, On-Demand and Surgery Patients with von Willebrand Disease* System Organ Class (SOC) Adverse Reaction Number of Subjects (%)†
(n=80)- *
- These trials were conducted using VONVENDI (recombinant VWF) and, when necessary, ADVATE [Antihemophilic factor (recombinant)], a recombinant factor VIII.
- †
- Percentages by subject were calculated using the number of all subjects who had the listed adverse events (AEs), including ALL AEs that are related.
Cardiac Disorders Tachycardia 1 (1.25%) Gastrointestinal Disorders Vomiting 3 (3.75%) Nausea 3 (3.75%) General Disorders and Administration Site Conditions Infusion site paresthesia 1 (1.25%) Chest discomfort 1 (1.25%) Skin and Subcutaneous Tissues Disorders Generalized pruritus 2 (2.50%) Vascular Disorders Hot flush 1 (1.25%) Hypertension 1 (1.25%) Deep vein thrombosis 1 (1.25%) Nervous System Disorders Dizziness 3 (3.75%) Vertigo 2 (2.50%) Dysgeusia 1 (1.25%) Tremor 1 (1.25%) Investigations Heart rate increase 1 (1.25%) Electrocardiogram T wave inversions 1 (1.25%) Single patient treated with Vonvendi in a clinical trial developed infusion-related reaction. This patient was previously exposed to Vonvendi without any symptoms. He developed chest discomfort and increased heart rate 3 minutes after the infusion was started. The patient was treated with supportive care and symptoms were resolved in 3 hours.
In the completed prophylaxis clinical study, twenty-two adult subjects of age 18 years and older receiving on-demand therapy or prophylactic therapy with plasma derived VWF prior to study entry received prophylactic treatment with VONVENDI during the study. The adverse drug reaction (ADR) terms are listed below (Table 6).
Table 6: Summary of Adverse Reactions from Completed Prophylaxis Clinical Trial in Patients with von Willebrand Disease* System Organ Class (SOC) Adverse Reaction Total (N=22)
n (%)†N=Total number of subjects in the Safety Analysis Set within each column.
n=Number of subjects who had at least one event in the category.- *
- These trials were conducted using VONVENDI (recombinant VWF) and, when necessary, ADVATE [Antihemophilic factor (recombinant)], a recombinant factor VIII.
- †
- Percentages by subject were calculated using the number of all subjects who had the listed adverse events (AEs).
- ‡
- Reported as possibly related by the investigator.
Nervous System Disorders Headache‡ 4 (18.2%) Musculoskeletal and Connective Tissue Disorders Arthralgia 3 (13.6%) Investigations ALT increased 2 (9.1%) AST increased 1 (4.5%) Cardiac Disorders Supraventricular tachycardia 1 (4.5%) Ventricular extrasystoles 1 (4.5%) Gastrointestinal Disorders Diarrhea 1 (4.5%) Skin and Subcutaneous Tissue Disorders Purpura 1 (4.5%) Rash pruritic 1 (4.5%) General Disorders and Administration Site Conditions Injection site irritation 1 (4.5%) 6.2 Postmarketing Experience
The following adverse drug reactions (ADR) have been identified during post-approval use of VONVENDI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postmarketing ADRs reported in association with VONVENDI treatment include infusion-related reactions (IRR) which may be clinically manifested by symptoms such as tachycardia, flushing, rash, dyspnea, and blurred vision. In the postmarketing cases reported, the symptoms resolved, and the patients fully recovered within 4 hours after stopping the infusion.
Anaphylactic reaction has been reported with use of Vonvendi in the postmarketing setting.
6.3 Immunogenicity
The immunogenicity of VONVENDI was assessed in clinical trials by assessing the development of neutralizing antibodies against VWF and FVIII, as well as binding antibodies against VWF, Furin, Chinese hamster ovary (CHO) protein and mouse IgG. Of the 100 subjects who received VONVENDI in the clinical trials, one subject who was treated with VONVENDI perioperatively developed treatment-emergent binding antibodies against VWF following a surgery, for whom no adverse events or lack of hemostatic efficacy was reported. No binding antibodies against potential impurities such as rFurin, CHO-protein or mouse IgG developed after treatment with VONVENDI.
Two subjects included in the study had pre-existing high-titer specific binding antibodies against VWF. The high-titer binding anti-VWF antibodies were associated with a significantly decreased VWF:Ag activity post-infusion of either plasma derived VWF (pdVWF) or rVWF and consequently, the decreased activity of VWF:RCo, VWF:CB and FVIII:C. This finding indicates the potential clinical significance of pre-existing binding (non-neutralizing) antibodies: VWD patients previously treated with pdVWF concentrates may be at risk to express a pre-existing binding antibody against VWF prior to first exposure to rVWF which could potentially result in a decreased hemostatic response to rVWF. Such patients could be managed clinically by administration of higher doses of rVWF based on the PK data for each individual patient.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Adequate and well-controlled studies with VONVENDI have not been conducted in pregnant women. Animal developmental and reproductive toxicity studies have not been conducted with VONVENDI. It is not known whether VONVENDI can cause fetal harm when administered to a pregnant woman or whether it can affect reproduction capacity.
The background risk of major birth defects and miscarriage in the indicated population is unknown; however, in the US population, the estimated background risk of major birth defects and miscarriage is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of VONVENDI in human milk, the effects on the breastfed infant, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VONVENDI and any potential adverse effects on the breastfed infant from VONVENDI or from the underlying maternal condition.
-
11 DESCRIPTION
VONVENDI is a purified rVWF expressed in Chinese Hamster Ovary (CHO) cells. VONVENDI is produced and formulated without the addition of any exogenous raw materials of human or animal origin in the cell culture, purification, or formulation of the final product. Proteins present in the final container product other than rVWF are trace quantities of mouse immunoglobulin (IgG, from the immunoaffinity purification), host cell (i.e., CHO) protein, rFurin (used to further process rVWF), and recombinant factor VIII (rFVIII).
Von Willebrand factor is a large multimeric glycoprotein that is normally found in plasma, and stored as ultra-large multimers in alpha-granules of platelets and intracellular organelles known as Weibel-Palade bodies, prior to secretion into the blood.1 Once the VWF is released to the blood stream and in contact with ADAMTS13 (a proteolytic enzyme in blood), it is cleaved to smaller sizes that can be detected with SDS agarose gels as multimer bands, representing the various species of VWF within the circulation. VONVENDI is rVWF that contains ultra-large multimers in addition to all of the multimers found in plasma as it is not exposed to proteolysis by ADAMTS13 during the manufacturing process.
VONVENDI is formulated as a sterile, non-pyrogenic, white to off-white friable powder for intravenous injection after reconstitution. VONVENDI in a single-dose vial contains nominally 650 or 1300 IU VWF:RCo.
The product contains no preservative. When reconstituted with the provided Sterile Water for Injection the final solution contains the following stabilizers and excipients (Table 7) in targeted amounts:
Table 7: Concentration of Stabilizer and Excipient after Reconstitution Stabilizer and Excipient Targeted Concentration for Nominal Strengths (650, 1300 IU) Tri-Sodium Citrate-dihydrate 15 mM Glycine 15 mM Mannitol 20 g/L Trehalose-dihydrate 10 g/L Polysorbate 80 0.1 g/L Each vial of VONVENDI is labeled with the specific number of units of VWF:RCo expressed in IU, which are based on the current World Health Organization (WHO) standard for VWF concentrate. After reconstitution of the lyophilized powder and filtration/withdrawal into a syringe, all dosage strengths yield a clear, colorless solution, free from particles.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In VWD patients, VONVENDI acts 1) to promote hemostasis by mediating platelet adhesion to damaged vascular sub-endothelial matrix (e.g., collagen) and platelet aggregation, and 2) as a carrier protein for factor VIII, protecting it from rapid proteolysis. The adhesive activity of VWF depends on the size of its multimers, with larger multimers being the most effective in supporting interactions with collagen and platelet receptors.1 The binding capacity and affinity of VONVENDI to factor VIII in plasma is comparable to that of endogenous VWF, allowing for VONVENDI to reduce factor VIII clearance.2
12.2 Pharmacodynamics
The PD of VWF following prophylactic treatment with VONVENDI were investigated in a clinical trial. In Prior OD adult subjects with Type 3 VWD, a single infusion of VONVENDI led to an increase of FVIII:C with peak levels observed approximately 24 hours post-infusion (mean [SD] dose: 50.2 [3.33] IU/kg). After 1-year repeat infusions of 50 ± 10 IU/kg VONVENDI twice weekly, the median (range) pre-dose FVIII:C increased from 2.0 (2.0 to 4.0) IU/dL (baseline after wash-out, N=10) to 10.5 (6.0 to 31.0) IU/dL (Month 12, N=8). The pre-dose FVIII:C (median [range] ≥10.5 [1.0 to 148] IU/dL) were observed over the prophylactic visits at Months 1, 2, 3, 6, 9 and 12. At Month 12, the mean (SD) Cmax and AUC(0-96 hours) of FVIII:C were 106 (37.2) IU/dL and 5962 (2671) IU*h/dL (N=8), respectively.
12.3 Pharmacokinetics
The PK profile of VONVENDI was determined based on data analyses from two clinical trials by assessment of VWF:RCo, VWF:Ag, and VWF:CB following a single dose. Subjects were evaluated in the non-bleeding state.
Table 8 below summarizes the PK parameters of VONVENDI after infusions of 50 IU/kg (PK50) or 80 IU/kg VWF:RCo (PK80) VONVENDI.
Table 8: Pharmacokinetic Assessment of VWF:RCo Following Single-Dose Infusion Parameter (unit) PK50 VONVENDI with ADVATE* PK50 VONVENDI PK80 VONVENDI Mean (SD)
Min; MaxMean (SD)
Min; MaxMean (SD)
Min; MaxAUC(0-inf) = area under plasma concentration-time curve from time 0 hour to infinite time post-infusion; IR = incremental recovery; CL = clearance; t1/2 = half-life. - *
- This study was conducted using VONVENDI (recombinant VWF) and ADVATE [Antihemophilic factor (recombinant)], a recombinant factor VIII.
T1/2 19.3 (10.99) 22.6 (5.34) 19.1 (4.32) (h) 10.8; 51.2 17.0; 37.2 11.8; 28.0 CL 0.04 (0.028) 0.02 (0.005) 0.03 (0.009) ([dL/kg]/h) 0.01; 0.16 0.02; 0.04 0.02; 0.05 IR 1.7 (0.62) 1.9 (0.41) 2.0 (0.39) ([IU/dL]/([IU/kg]) 1.0; 3.6 1.2; 2.7 1.4; 2.9 AUC0-inf 1541.4 (554.31) 2105.4 (427.51) 2939.0 (732.72) (IU*h/dL) 173.8; 2862.0 1334.0; 2813.3 1507.8; 4121.1 AUC0-inf/Dose 33.4 (13.87) 42.1 (8.31) 36.8 (8.97) ([IU*h/dL]/[IU/kg]) 6.4; 70.4 27.8; 54.8 18.8; 50.4 The PK of VWF following prophylactic treatment with VONVENDI were investigated in a clinical trial. Steady state PK parameters of VWF:RCo are presented in Table 9 for subjects with Type 3 VWD who were previously treated on-demand (OD) with any VWF product prior to study entry (Prior OD group) and subjects who were previously treated prophylactically with a plasma derived VWF product (Switch group) prior to study enrollment. The pharmacokinetics of VWF following single and multiple dosing (at Month 12) were similar in the Prior OD group.
Table 9: Steady State Pharmacokinetic Assessment of VWF:RCo (Month 12; Subjects with Type 3 VWD) Parameter
(unit)Prior OD Group
N=8
Mean (SD)
Min; MaxSwitch Group
N=6
Mean (SD)
Min; MaxAUC(0-96 hours) = area under plasma concentration-time curve from time 0 to 96 hours post-infusion; Cmax = maximum plasma concentration; IR = incremental recovery; CL = clearance; t1/2 = half-life; dose regimens for subjects with Type 3 VWD in Prior OD group were 41 to 56 IU/kg and for subjects with Type 3 VWD in Switch group were 24 to 63 IU/kg. T1/2 16.5 (4.13)* 14.1 (6.13)† (h) 10.9; 22.4 9.4; 22.9 CL 0.04 (0.012)* 0.04 (0.014)† ([dL/kg]/h) 0.02; 0.05 0.02, 0.06 IR at Cmax 1.8 (0.58) 1.8 (0.25) ([IU/dL] / [IU/kg]) 0.9, 2.7 1.6, 2.2 Cmax 86.4 (34.2) 90.6 (33.7) (IU/dL) 41.6, 149.0 46.7, 141.0 Cmax/Dose 1.8 (0.58) 1.8 (0.25) ([IU/dL]/[IU/kg]) 0.9, 2.7 1.6; 2.2 AUC(0-96 hours) 1199 (760) 1662 (677)‡ (IU*h/dL) 460, 2940 1230, 2440 AUC(0- 96 hours)/Dose 24.3 (13.40) 27.5 (9.74)‡ ([IU*h/dL]/[IU/kg]) 9.8, 53.0 21.7, 38.7 -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In vitro and in vivo genotoxicity studies indicated no mutagenic potential for VONVENDI. Long-term animal studies to assess the carcinogenic potential of VONVENDI were not performed. Animal studies evaluating the developmental and reproductive toxicity of VONVENDI were not conducted.
-
14 CLINICAL STUDIES
On-Demand Treatment and Control of Bleeding Episodes
Hemostatic efficacy of VONVENDI was assessed in a multicenter, open-label trial investigating different dosing strategies with and without rFVIII for on-demand treatment and control of bleeding episodes in adults (age 18 years and older) diagnosed with von Willebrand disease. In this trial, all subjects requiring rFVIII received ADVATE [Antihemophilic factor (recombinant)].
Bleeding episodes were treated initially with an infusion of VONVENDI and ADVATE at a ratio of 1.3:1 respectively (i.e., 30% more VONVENDI than ADVATE), and subsequently with VONVENDI with or without ADVATE, based on FVIII:C levels. The aim of the initial dose of VONVENDI with ADVATE was to achieve target plasma levels of greater than 60 IU/dL (60%) VWF:RCo and greater than 40 IU/dL (40%) of FVIII:C.
A total of 193 bleeding episodes were reported in 22/37 subjects exposed to VONVENDI for on-demand treatment. Demographic and baseline characteristics are listed in Table 10.
Table 10: Demographic and Baseline Characteristics Parameter Category Exposed Subjects
n=37
n (%)Treated Subjects
n=22
n (%)Gender Male 17 (45.9) 10 (45.5) Female 20 (54.1) 12 (54.5) Age Median (years) 37.0 28.0 Race Caucasian 32 (86.5) 20 (90.9) Asian 5 (13.5) 2 (9.1) Ethnicity Hispanic or Latino 2 (5.4) 2 (9.1) Not Hispanic or Latino 35 (94.6) 20 (90.9) VWD Type 1 2 (5.4) 0 (0.0) 2A 5 (13.5) 4 (18.2) 2B 0 (0.0) 0 (0.0) 2M 0 (0.0) 0 (0.0) 2N 1 (2.7) 1 (4.5) 3 29 (78.4) 17 (77.3) The primary efficacy endpoint was the number of subjects with treatment success for control of bleeding episodes. Treatment success was defined as a mean efficacy rating score of less than 2.5 for all bleeding episodes in a subject treated with VONVENDI (with or without ADVATE) during the trial period. The efficacy rating was assessed using a pre-specified 4-point rating scale comparing the prospectively estimated number of infusions needed to treat the bleeding episodes as assessed by the investigator to the actual number of infusions administered. The definitions for each of the 4-point rating scales are provided in Table 11.
Table 11: Definitions of 4-Point Rating Scales Rating Minor and Moderate Bleeding Events Major Bleeding Events Excellent
(= 1)Actual number of infusions ≤ estimated number of infusions required to treat that bleeding episode.
No additional VWF coagulation factor containing product required.Actual number of infusions ≤ estimated number of infusions required to treat that bleeding episode.
No additional VWF coagulation factor containing product required.Good
(= 2)1 to 2 infusions greater than estimated required to control that bleeding episode.
No additional VWF coagulation factor containing product required.<1.5× infusions greater than estimated required to control that bleeding episode.
No additional VWF coagulation factor containing product required.Moderate
(= 3)3 or more infusions greater than estimated used to control that bleeding event.
No additional VWF coagulation factor containing product required.≥1.5× more infusions greater than estimated used to control that bleeding event.
No additional VWF coagulation factor containing product required.None
(= 4)Severe uncontrolled bleeding or intensity of bleeding not changed.
Additional VWF coagulation factor containing product required.Severe uncontrolled bleeding or intensity of bleeding not changed.
Additional VWF coagulation factor containing product required.Secondary efficacy measures were the number of treated bleeding episodes with an efficacy rating of 'excellent' or 'good', the number of infusions and number of units of VONVENDI, administered with or without ADVATE, per bleeding episode.
The primary efficacy assessment excluded subjects with GI bleeds (n=2), and subjects in whom the number of infusions to control a bleeding episode was estimated retrospectively (n=2). The rate of subjects (n=18) with treatment success was 100% (95% CI, 81.5 to 100). Sensitivity analyses of treatment success for bleeding episodes including GI bleeds and those bleeding episodes for which the investigator had to make retrospective assessment of the number of infusions required (n=22: 17 with type 3 VWD, 4 with type 2A VWD and 1 with type 2N VWD) confirmed the primary analysis, with a 100% treatment success rate for each scenario.
All bleeding episodes treated with VONVENDI and ADVATE or VONVENDI alone were controlled with an efficacy rating of excellent (96.9%) or good (3.1%). Control of bleeding episodes was consistent across all degrees of severity.
For an overview of hemostatic efficacy by bleeding severity and number of infusions required to treat a bleeding episode, refer to Table 12.
Table 12: Number of Infusions by Severity of Bleeding Episodes* Number of Infusions per Bleed Severity of Bleeding Episodes Minor
n (%)
n=122Moderate
n (%)
n=61Major/Severe
n (%)
n=7Unknown
n (%)
n=2All
n (%)
n=192- *
- One subject received plasma-derived VWF for one bleeding episode for the 3rd infusion and therefore was excluded from Table 12.
1 113 (92.6%) 41 (67.2%) 1 (14.3%) 2 (100%) 157 (81.8%) 2 8 (6.6%) 13 (21.3%) 4 (57.1%) 0 (0.0) 25 (13.0%) 3 1 (0.8%) 6 (9.8%) 2 (28.6%) 0 (0.0) 9 (4.7%) 4 0 (0.0) 1 (1.6%) 0 (0.0) 0 (0.0) 1 (0.5%) Median 1 1 2 1 1 Range 1 to 3 1 to 4 1 to 3 1 to 1 1 to 4 The median cumulative dose of VONVENDI administered per bleeding episode (with or without ADVATE) was 48.2 IU/kg (90% CI, 43.9 to 50.2) IU/kg. In relation to bleeding severity, the median cumulative dose to treat a bleeding episode was 43.3 (range, 25.2 to 158.2) IU/kg for minor bleeding episodes (n=122), 52.7 (range, 23.8 to 184.9) IU/kg for moderate bleeding episodes (n=61), 100 (range, 57.5 to 135) IU/kg for major bleeding episodes (n=7).
Table 13 summarizes data obtained for number of infusions and efficacy rating per bleeding episode by location.
Table 13: Efficacy by Bleeding Episode Location Bleeding Episodes by Location (n) Median Number of Infusions (Range) Rating (%) Joint (n=59) 1 (1 to 3) Excellent (96.6%) Good (3.4%) GI (n=6) 1 (1 to 2) Excellent (83.3%) Good (16.7%) Mucosal: Genital Tract Female (n=32) 1 (1 to 2) Excellent (96.9%) Good (3.1%) Mucosal: Nasopharyngeal (n=42) 1 (1 to 2) Excellent (97.6%) Good (2.4%) Mucosal: Mouth and Oral Cavity (n=26) 1 (1 to 4) Excellent (100%) Good (0%) Perioperative Management of Bleeding
Hemostatic efficacy of VONVENDI was assessed in a prospective, open-label, multicenter trial to evaluate efficacy and safety of VONVENDI with or without ADVATE in elective surgical procedures in adults (age 18 years and older) diagnosed with severe VWD and the subjects were followed for 14 days after surgery.
A total of 15 VWD subjects completed the trial and 93% of the subjects were less than 65 years old (range, 20 to 70 years), of whom 53.3% were females and 53% (8/15) were type 3 VWD patients. Out of 15 subjects, 10 subjects underwent major surgeries and five subjects underwent minor surgeries.
Major surgeries included orthopedic surgeries: total hip replacement, total knee replacement, knee endoprosthesis, ankle prosthesis, anterior cruciate ligament surgery and meniscectomy. Other major surgeries included laparoscopic cholecystectomy, laparoscopic cystectomy and complex dental extractions. Minor surgeries/procedures included nasopharyngoscopy, dental extractions, colonoscopy and radioisotope synovectomy.
All subjects were administered a 12 to 24 hour preoperative dose of 40 to 60 IU/kg of VONVENDI to increase the factor VIII levels to target levels. Within 3 hours prior to surgery, the subjects' FVIII:C levels were assessed to ensure that target of 30 IU/dL for minor surgeries and 60 IU/dL for major surgeries was achieved. Within 1 hour prior to surgery, subjects received a dose of VONVENDI. ADVATE (recombinant Factor VIII) was administered based on FVIII:C levels performed 3 hours prior to surgery. VWF and factor VIII Incremental recovery were used to guide the initial and subsequent doses.
Six of the 10 subjects undergoing major surgery received protocol-specified loading dose. It should be noted that the protocol-specified loading dose was based on VWF:RCo levels assessed prior to the 12 to 24 hour preoperative dose. Four of 10 subjects undergoing major surgery and four out of five subjects undergoing minor surgery received a loading dose of VONVENDI based on VWF:RCo assessed prior to loading dose and after administration of the 12 to 24 hour preoperative dose. Unlike the protocol-specified loading dose based on the levels assessed prior to the preoperative dose between 12 to 24 hours of the surgery, the loading doses in these eight subjects were calculated based on VWF:RCo levels after a preoperative dose, and were therefore lower doses than protocol-specified loading dose. No differences in safety or efficacy were noted between the two groups.
The primary outcome measure was the overall hemostatic efficacy assessed 24 hours after the last perioperative VONVENDI infusion or at completion of study visit whichever occurred earlier using a 4-point ordinal efficacy scale outlined in Table 14 ("excellent", "good", "moderate" and "none") based on estimated expected versus actual blood loss, transfusion requirements and postoperative bleeding and oozing. A rating of excellent or good was required to declare the outcome a success.
Table 14: Primary Efficacy Assessment Rating Overall Assessment of Hemostatic Efficacy 24 Hours after Last Perioperative IP Infusion or at Day 14 Completion Visit (Whatever Occurs Earlier) Excellent (1) Intra- and postoperative hemostasis achieved with rVWF with or without ADVATE was as good or better than that expected for the type of surgical procedure performed in a hemostatically normal subject. Good (2) Intra- and postoperative hemostasis achieved with rVWF with or without ADVATE was probably as good as that expected for the type of surgical procedure performed in a hemostatically normal subject. Moderate (3) Intra- and postoperative hemostasis with rVWF with or without ADVATE was clearly less than optimal for the type of procedure performed but was maintained without the need to change the rVWF concentrate. None (4) Subject experienced uncontrolled bleeding that was the result of inadequate therapeutic response despite proper dosing, necessitating a change of rVWF concentrate. Overall hemostatic efficacy for major and minor surgeries was 100% (15/15) with a 90% confidence interval of 81.9% to 100%. It was excellent for 60% of surgeries and good for 40% of surgeries.
Intraoperative hemostatic efficacy was a secondary endpoint. For major and minor surgeries, it was 100% with a 90% confidence interval of 81.9% to 100%. It was excellent for 73.3% of surgeries and good for 26.7% of surgeries.
For details regarding hemostatic efficacy for minor and major surgery, see Table 15.
Table 15: Overall Hemostatic Efficacy Type of Surgery Excellent
9/15 (60%)Good
6/15 (40%)Moderate
0/15 (0.0%)Total
N=15Minor 4 1 0 5 Major 5 5 0 10 Dosing was individualized based on incremental recovery results performed before surgery.
Mean total 12 to 24 preoperative dose was 50.9 IU/kg (median 55.0 IU/kg; range, 36.1 to 59.9 IU/kg).
Mean total loading dose (1 hour preoperative dose) per infusion was 38.6 IU/kg (median 35.8 IU/kg; range, 8.0 to 82.7 IU/kg). Major surgeries required a mean loading dose of 42.8 IU/kg (median 37.6 IU/kg; range, 15.7 to 82.7 IU/kg) in comparison with a mean loading dose of 30.2 IU/kg (median 34.2 IU/kg; range, 8.0 to 46.4 IU/kg) for minor surgeries.
For subjects treated with VONVENDI (with or without ADVATE), the median total postoperative dose within the first 7 days after surgery was 114.2 IU/kg with a range of 23.8 to 318.9 IU/kg (n=13) and 76.2 IU/kg with a range of 23.8 to 214.4 IU/kg for the next 7 postoperative days (n=8).
Routine Prophylaxis to Reduce the Frequency of Bleeding Episodes in Patients with Severe Type 3 VWD Receiving On-Demand Therapy
VONVENDI was studied in a prospective, single arm, open-label, international multicenter study to evaluate efficacy, safety, PK and pharmacodynamics (PD) of prophylactic treatment in reducing the frequency of bleeding episodes in adult subjects (age 18 years and older) diagnosed with VWD.
Subjects were enrolled into two treatment arms based on the treatment they received for management of bleeding prior to study entry consisting of a Prior OD group in which subjects received on-demand (OD) treatment only and the Switch group in which subjects had received prophylactic treatment with plasma derived VWF (pdVWF). Efficacy was assessed based on the median annualized bleeding rate for all bleeds, spontaneous bleeds and joint bleeds and was based on descriptive statistics.
Twenty-two efficacy evaluable subjects received a median of 92.5 doses, 12 of these subjects had previously received on demand treatment (Prior OD group) and 10 subjects previously received prophylactic treatment with pdVWF (Switch group) prior to enrolling into this study. Nine subjects in the OD group and 8 subjects in the Switch group completed the study which required 12 months of treatment.
Median age of subjects in the study was 34 years (18 - 77 years), 54.5% were male and 96% of subjects were white and 86.4% were non-Hispanic or Latino. Of the 12 subjects in the Prior OD group, one had severe Type 1, one had severe Type 2 VWD, and ten had severe Type 3 VWD. Of the 10 subjects in the Switch group, one had severe Type 1, one had severe Type 2 VWD, and eight had severe Type 3 VWD.
Subjects in the Prior OD group began treatment at 50 ± 10 IU/kg per infusion twice weekly. One subject in the Prior OD group required dose adjustments for management of breakthrough bleeding. The majority of subjects (9/10) with Type 3 VWD in the Prior OD group received twice weekly dosing with Vonvendi and had a median maximum dose of 55.9 (IU/kg).
The study did not adequately demonstrate efficacy of Vonvendi as prophylactic treatment to reduce bleeding events in subjects with Type 3 VWD receiving prophylactic therapy prior to study entry (Switch group).
The efficacy parameters of Vonvendi as prophylactic treatment in subjects with Type 3 VWD receiving on-demand treatment prior to study entry is provided in Table 16. The median percentage change of ABRs from historical to on-study were: for all bleeds -54.7%, for spontaneous bleeds -75.9%, and for joint bleeds -100.0%.
Table 16: Efficacy of Routine Prophylaxis with Vonvendi in Prior OD Subjects with Severe Type 3 VWD (N=10) Type or Site of Bleeding Event Number of BEs Historical/On-Study Historical Median ABR*
(Min, Max)On-Study Median ABR*
(Min, Max)All Bleeds† 201/38 5.0
(3.0, 159.0)2.3
(0, 157.9)Spontaneous Bleeds‡ 195/33 3.5
(3.0, 158.0)1.0
(0.0, 157.9)Joint Bleeds† 23/3 2.0
(0.0, 7.0)0.0
(0.0, 1.9) -
15 REFERENCES
- Stockschlaeder M, Schneppenheim R, Budde U, Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis 2014, 25:206-216.
- Turecek PL, Mitterer A, Matthiessen HP, Gritsch H, Varadi K, Siekmann J, Schnecker K, Plaimauer B, Kaliwoda M, Purtscher M, Woehrer W, Mundt W, Muchitsch EM, Suiter T, Ewenstein BM, Ehrlich HJ, Schwarz HP, Development of a plasma- and albumin-free recombinant von Willebrand factor. Haemastaseologie 2009; 29 (Suppl 1): 32-38.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
VONVENDI is packaged with Sterile Water for Injection (sWFI), one Mix2Vial reconstitution device, one full prescribing physician insert, and one patient insert.
VONVENDI is available in single-dose vials that contain the following product strengths:
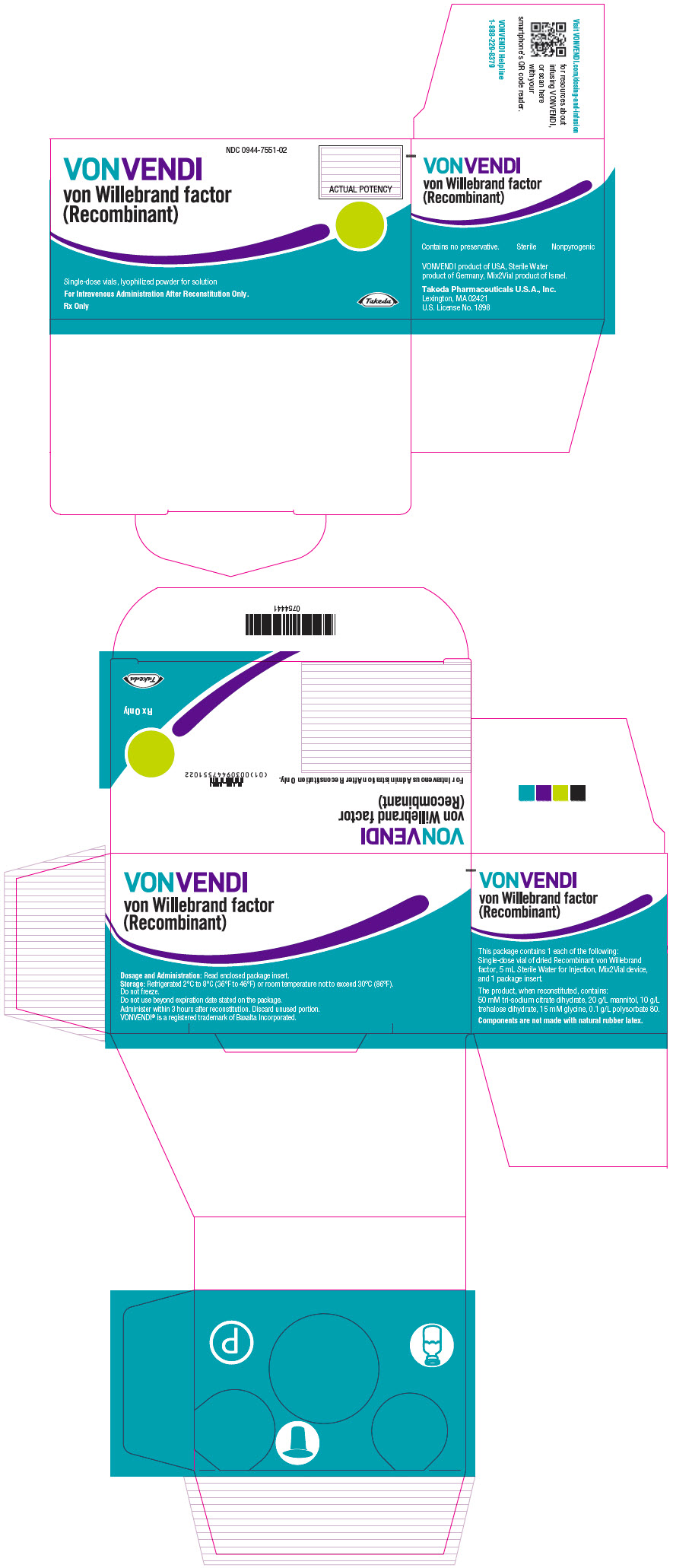
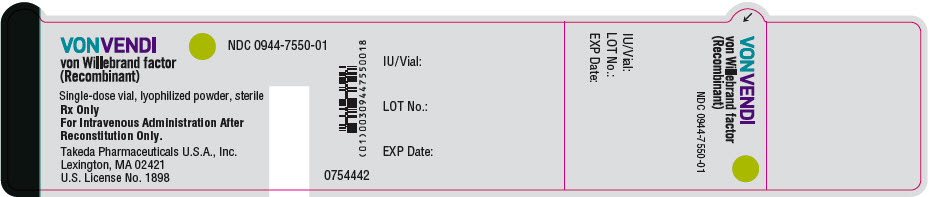
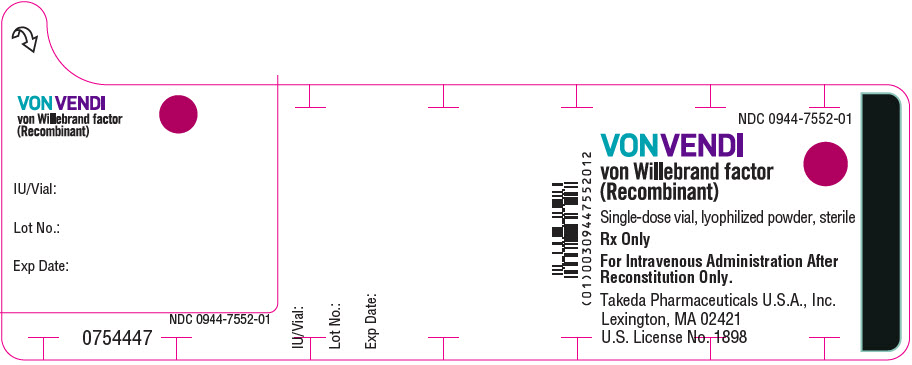
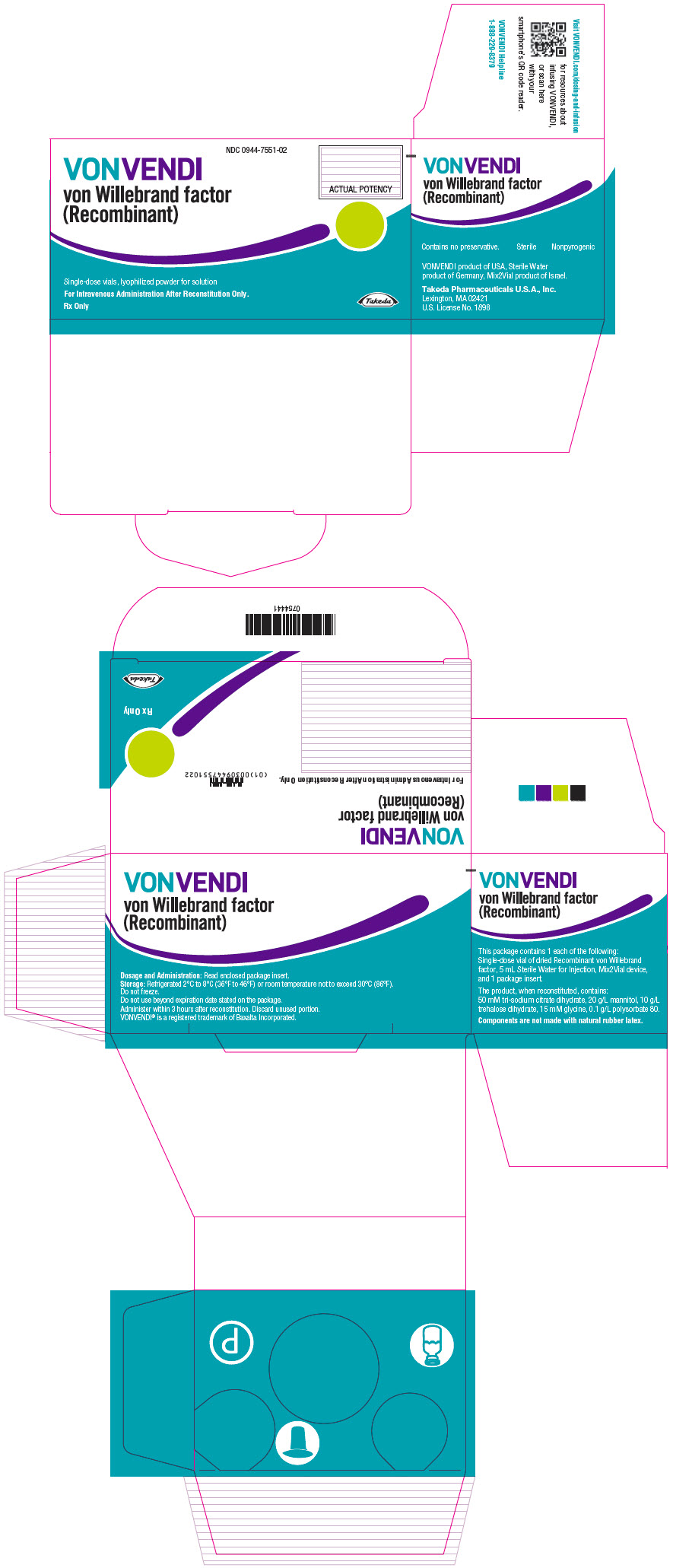
Color Code VWF:RCo Potency Range Carton NDC sWFI fill size Green 450–850 IU per vial 0944-7551-02 5 mL Dark Red 900–1700 IU per vial 0944-7553-02 10 mL The actual von Willebrand factor activity in international units is printed on the label of each VONVENDI vial and carton.
Components are not made with natural rubber latex.
Storage and Handling
- Store at refrigerated temperature 2°C to 8°C (36°F to 46°F) or room temperature not to exceed 30°C (86°F).
- Do not freeze.
- Store in the original box and protect from extreme exposure to light.
- Do not use beyond the expiration date printed on the VONVENDI vial label or carton.
- Do not use if the solution in the syringe is cloudy or contains flakes or particles after filtration from the vial into the syringe.
- Use reconstituted product immediately or within 3 hours after reconstitution.
- Discard any unused reconstituted product after 3 hours.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient:
- To read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- About early signs of hypersensitivity reactions, including anaphylactic shock, generalized urticaria, angioedema, chest tightness, hypotension, shock, lethargy, nausea, vomiting, paresthesia, pruritus, restlessness, wheezing and/or acute respiratory distress. Advise patients to discontinue use of the product if these symptoms occur and seek immediate emergency treatment with resuscitative measures.
- To contact their physician or treatment center for further treatment and/or assessment if they experience a lack of clinical response to von Willebrand factor therapy, as this may be a manifestation of an inhibitor.
- To consult with their physicians or healthcare provider prior to travel. While traveling, advise patients to bring an adequate supply of VONVENDI based on their current regimen of treatment.
-
SPL UNCLASSIFIED SECTION
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898VONVENDI® is a registered trademark of Baxalta Incorporated.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
Mix2Vial® is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.
Patented: see www.takeda.com/en-us/patents
VON355
-
PATIENT PACKAGE INSERT
Patient Information
VONVENDI
[von Willebrand factor (recombinant)]This Patient Information has been approved by the U.S. Food and Drug Administration. This leaflet summarizes important information about VONVENDI. Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about VONVENDI. If you have any questions after reading this, ask your healthcare provider.
Do not attempt to do an infusion to yourself unless you have been taught how by your healthcare provider or hemophilia center.
You must carefully follow your healthcare provider's instructions regarding the dose and schedule for infusing VONVENDI so that your treatment will work best for you.What is VONVENDI?
VONVENDI is a recombinant medicine used to replace low levels or not properly working von Willebrand factor in people with von Willebrand disease. Von Willebrand disease is an inherited bleeding disorder in which blood does not clot normally.
VONVENDI is used in adults (age 18 years and older) diagnosed with von Willebrand disease to:- treat and control bleeding episodes
- prevent excessive bleeding during and after surgery
- reduce the number of bleeding episodes when used regularly (prophylaxis)
Who should not use VONVENDI?
You should not use VONVENDI if you:- Are allergic to any ingredients in VONVENDI.
- Are allergic to mice or hamsters.
What should I tell my healthcare provider before I use VONVENDI?
You should tell your healthcare provider if you:- Have or have had any medical problems.
- Take any medicines, including prescription and non-prescription medicines, such as over-the-counter medicines, supplements or herbal remedies.
- Have any allergies, including allergies to mice or hamsters.
- Are breastfeeding. It is not known if VONVENDI passes into your milk and if it can harm your baby.
- Are pregnant or planning to become pregnant. It is not known if VONVENDI can harm your unborn baby.
- Have been told that you have inhibitors to von Willebrand factor (because VONVENDI may not work for you).
- Have been told that you have inhibitors to blood coagulation factor VIII.
How should I use VONVENDI?
VONVENDI is given directly into the bloodstream. Your first dose of VONVENDI for each bleeding episode may be administered with a recombinant factor VIII (factor VIII not produced from plasma) as instructed by your healthcare provider.
Your healthcare provider will instruct you whether additional doses of VONVENDI with or without recombinant factor VIII are needed.
You may infuse VONVENDI at a hemophilia treatment center, at your healthcare provider's office or in your home. You should be trained on how to do infusions by your healthcare provider or hemophilia treatment center. Many people with von Willebrand disease learn to infuse VONVENDI by themselves or with the help of a family member.
Your healthcare provider will tell you how much VONVENDI to use based on your weight, the severity of your von Willebrand disease, and where you are bleeding.
Call your healthcare provider right away if your bleeding does not stop after taking VONVENDI.
Use the reconstituted product (after mixing dry product with the supplied sterile water) immediately. If not, store at room temperature not to exceed 25°C (77°F) for up to 3 hours. Discard after 3 hours.What are the possible side effects of VONVENDI?
You can have an allergic reaction to VONVENDI.
Call your healthcare provider right away and stop treatment if you get a rash or hives, itching, tightness of the throat, chest pain or tightness, difficulty breathing, lightheadedness, dizziness, nausea or fainting.
Side effects that have been reported with VONVENDI include: headache, nausea, vomiting, tingling or burning at infusion site, chest discomfort, dizziness, joint pain, joint injury, increased liver enzyme level in blood, hot flashes, itching, high blood pressure, muscle twitching, unusual taste, blood clots and increased heart rate. These are not all the possible side effects with VONVENDI. You can ask your healthcare provider for information that is written for healthcare professionals.
Tell your healthcare provider about any side effects that bother you or do not go away.What are the VONVENDI dosage strengths?
VONVENDI with Sterile Water for Injection and Mix2Vial reconstitution device comes in two different dosage strengths.Green: Dosage strength of approximately 650 International Units (450–850 IU) (reconstituted with 5 mL of sterile water). Dark Red: Dosage strength of approximately 1300 International Units (900–1700 IU) (reconstituted with 10 mL of sterile water). - The actual strength will be printed on the vial label and on the box. Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider.
- Always check the expiration date printed on the box.
- Do not use the product after the expiration date printed on the box.
How do I store VONVENDI? - Store at refrigerated temperature 2°C to 8°C (36°F to 46°F) or room temperature not to exceed 30°C (86°F).
- Do not freeze.
- Store VONVENDI in the original box and protect from extreme exposure to light.
- Do not use beyond the expiration date.
- Do not use the product if the solution is not clear or if it contains particles after your draw it into the syringe.
- Use reconstituted product (after mixing dry product with supplied sterile water) immediately or within 3 hours of reconstitution.
- Store the reconstituted product at room temperature not to exceed 25°C (77°F) for up to 3 hours. Discard any unused reconstituted product after 3 hours.
What else should I know about VONVENDI and von Willebrand Disease?
Your body can form inhibitors to von Willebrand factor or factor VIII. An inhibitor is part of the body's normal defense system. If you form inhibitors, they may stop VONVENDI or FVIII from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to von Willebrand factor or factor VIII.
Medicines are sometimes prescribed for purposes other than those listed here. Do not use VONVENDI for a condition for which it is not prescribed. Do not share VONVENDI with other people, even if they have the same symptoms that you have.Resources at Takeda available to the patients:
For more product information on VONVENDI, please visit www.VONVENDI.com or call 1-877-TAKEDA-7 (1-877-825-3327).
For information on additional Takeda patient resources, please visit www.VONVENDI.com.
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898
VONVENDI® is a registered trademark of Baxalta Incorporated.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
Mix2Vial® is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.
Patented: see www.takeda.com/en-us/patents
Revised: 3/2023 VON355 -
Instructions for UseVONVENDI[von Willebrand factor (recombinant)] (For intravenous use only)
For first dose for each bleeding episode, use with recombinant factor VIII as instructed by your physician.
- Always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using VONVENDI. If you are unsure of the procedures, please call your healthcare provider before using.
- Call your healthcare provider right away if bleeding is not controlled after using VONVENDI.
- Your healthcare provider will prescribe the VONVENDI dose that you should take. If this is your first infusion to control a new bleeding episode, and if recombinant factor VIII in needed, be sure you also have the correct dose of recombinant factor VIII as instructed by your physician.
- Your healthcare provider may need to take blood tests from time to time.
- Talk to your healthcare provider before traveling. Plan to bring enough VONVENDI for your treatment during this time.
- Dispose of all materials, including any leftover reconstituted VONVENDI product, in an appropriate container.
See below for step-by-step instructions for reconstituting and administrating VONVENDI:
Reconstitution
- Prepare a clean flat surface and gather all the materials you will need for the infusion. Check the expiration date, and let the VONVENDI warm up to room temperature. Wash your hands and put on clean exam gloves. If infusing yourself at home, the use of gloves is optional.
- Remove the caps from the VONVENDI concentrate and diluent vials to expose the center of the rubber stoppers.
- Disinfect each stopper with a separate sterile alcohol swab (or other suitable sterile solution suggested by your healthcare provider or hemophilia center) by rubbing the stopper for several seconds and allow it to dry prior to use. Place the vials on a flat surface.
- Open the Mix2Vial device package by completely peeling away the lid, without touching the inside of the package. Do not remove the Mix2Vial device from the package.
- Turn the package with the Mix2Vial device upside down and place it over the top of the diluent vial. Firmly insert the blue plastic spike of the device into the center of the diluent vial stopper by pushing straight down. Grip the package at its edge and lift it off the Mix2Vial device. Be careful not to touch the clear plastic spike. The diluent vial now has the Mix2Vial device connected to it and is ready to be connected to the VONVENDI vial.
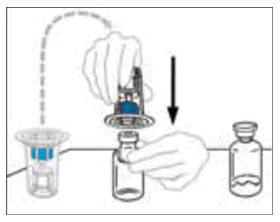
- To connect the diluent vial to the VONVENDI vial, turn the diluent vial over and place it on top of the vial containing VONVENDI concentrate. Fully insert the clear plastic spike into the VONVENDI vial stopper by firmly pushing straight down. This should be done right away to keep the liquid free of germs. The diluent will flow into the VONVENDI vial by vacuum. Verify that diluent transfer is complete. Do not use if vacuum has been lost.
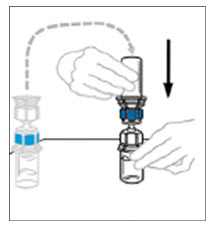
- Gently and continuously swirl the connected vials or allow the reconstituted product to sit for 5 minutes then gently swirl to ensure the powder is completely dissolved. Do not shake. Shaking will adversely affect the product. Do not refrigerate after reconstitution.
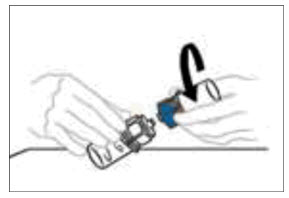
Note: Some flakes or particles may remain in the reconstituted vial. The filter included in the Mix2Vial device will remove extraneous flakes or particles, and the resulting solution in the syringe should be clear and colorless. Do not use the solution in the syringe if it is cloudy or contains flakes or particles after filtration from the vial into the syringe.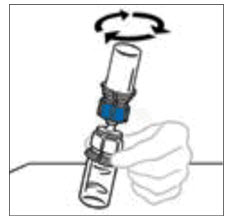
- Disconnect the two sides of the Mix2Vial from each other by holding the clear plastic side of the Mix2Vial device attached to the VONVENDI vial with one hand and the blue plastic side of the Mix2Vial device attached to the diluent vial with the other hand. Turn the blue plastic side counterclockwise and gently pull the two vials apart. Do not touch the end of the plastic connector attached to the VONVENDI vial containing the dissolved product. Place the VONVENDI vial on a flat work surface. Discard the empty diluent vial.
- Draw air into the empty, sterile disposable plastic syringe by pulling back on the plunger. The amount of air should equal the amount of reconstituted VONVENDI that you will withdraw from the vial.
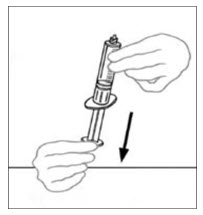
- Leaving the VONVENDI vial (containing the dissolved product) on your flat work surface, connect the syringe to the clear plastic connector by attaching and turning the syringe clockwise (Figure A). Hold the vial with one hand and use the other hand to push the entire amount of air from the syringe into the vial (Figure B). The required amount of product will not be drawn into the syringe if all the air is not pushed into the vial.
Figure A Figure B 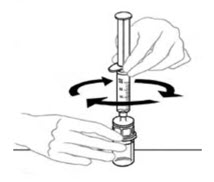
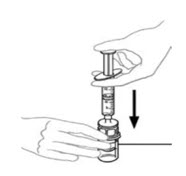
- Flip connected syringe and VONVENDI vial so the vial is on top. Be sure to keep the syringe plunger pressed in. Draw the VONVENDI into the syringe by pulling plunger back slowly. Do not push and pull solution back and forth between syringe and vial. Doing so may harm the integrity of the product. Inspect syringe visually for particulate matter; the solution should be clear and colorless in appearance. If flakes or particles are seen, do not use the solution and notify your healthcare provider.
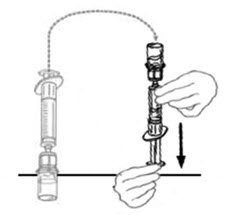
- When ready to infuse, disconnect the syringe by turning it counterclockwise.
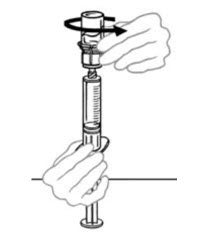
- If your dose requires more than one vial of VONVENDI, reconstitute each vial using a separate Mix2Vial device following the reconstitution steps above (2 to 8).
- Up to two vials of VONVENDI may be pooled into a single syringe. If you have a second vial of reconstituted VONVENDI:
- Disconnect the syringe with reconstituted solution carefully from the first vial of VONVENDI by turning the syringe counterclockwise. Do not touch exposed connector.
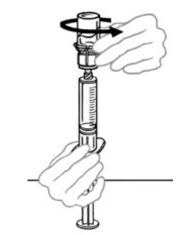
- Pull the plunger back to draw air into the syringe containing the first vial of reconstituted VONVENDI. The amount of air added should equal the amount of reconstituted VONVENDI you will withdraw from the second vial. Do not touch exposed connector.
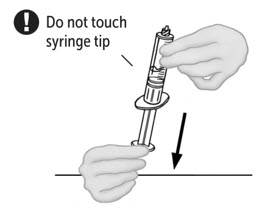
- Leave second vial of VONVENDI on flat surface and connect syringe by attaching to plastic connector and turning syringe clockwise.
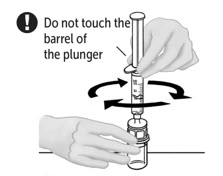
- Flip connected syringe and second vial of VONVENDI so vial is on top (Figure C). Hold the vial with one hand and use the other hand to slowly push air into vial by pressing the syringe plunger (Figure D). Do not push any fluid from the syringe into the vial.
Figure C Figure D 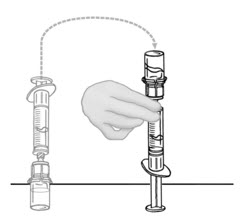
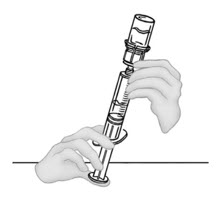
- Draw reconstituted VONVENDI from second vial of VONVENDI into syringe by slowly pulling back the plunger. Do not push any fluid from the syringe into the vial.
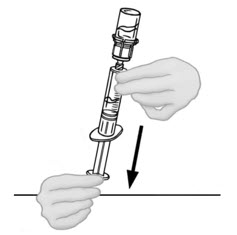
- Leave syringe attached to vial until ready to infuse to reduce risk of contamination. If your dose requires more than two vials of reconstituted VONVENDI, go back to Step 9 to draw your third vial into a NEW plastic syringe. No more than two vials of VONVENDI may be pooled into a single syringe. Pooling of more than two vials into a syringe may result in formation of filaments, which requires discarding of the solution in the syringe.
- Disconnect the syringe with reconstituted solution carefully from the first vial of VONVENDI by turning the syringe counterclockwise. Do not touch exposed connector.
- If you need to infuse recombinant factor VIII, reconstitute recombinant factor VIII as instructed in the package insert for that product. Do not administer your recombinant factor VIII until you have infused your complete dose of VONVENDI. Always follow your healthcare provider's specific directions.
Administration
- Attach the infusion needle to a syringe containing VONVENDI solution. For comfort, a winged (butterfly) infusion set is preferred. Point the needle up and remove any air bubbles by gently tapping the syringe with your finger and slowly and carefully pushing air out of the syringe and needle.
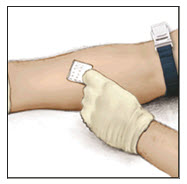
- Apply a tourniquet and get the injection site ready by wiping the skin well with a sterile alcohol swab (or other suitable sterile solution suggested by your healthcare provider or hemophilia center).
- Insert the needle into the vein and remove the tourniquet. Slowly infuse the VONVENDI. Do not infuse any faster than 4 mL per minute.
- Disconnect the empty syringe. If your dose requires multiple syringes, attach and administer each additional syringe of VONVENDI one at a time.
- Do not remove butterfly needle until all syringes have been infused and do not touch the Luer port that connects to the syringe.
- If your healthcare provider has prescribed recombinant factor VIII, administer recombinant factor VIII within 10 minutes after you have infused your complete dose of VONVENDI.
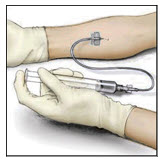
- Take the needle out of the vein and use sterile gauze to put pressure on the infusion site for several minutes.
- Do not recap the needle. Place the needle, syringe, and empty VONVENDI and diluent vial(s) in a hard-walled sharps container for proper disposal. Do not dispose of these supplies in ordinary household trash.
Important: Contact your healthcare provider or local hemophilia treatment center if you experience any problems.
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898VONVENDI® is a registered trademark of Baxalta Incorporated.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
Mix2Vial® is a registered trademark of West Pharma. Services IL, Ltd., a subsidiary of West Pharmaceutical Services, Inc.
Patented: see www.takeda.com/en-us/patents
Revised: 3/2023
VON355This Instructions for Use has been approved by the U.S. Food and Drug Administration.
- PRINCIPAL DISPLAY PANEL - Kit Carton - 650 IU
- PRINCIPAL DISPLAY PANEL - 650 IU Vial Label
-
PRINCIPAL DISPLAY PANEL - 5 mL Vial Label
5 mL
NDC 64764-515-50Takeda
Sterile Water for Injection, USP
for reconstitution of accompanying productDo not use unless clear. No antimicrobial agent or other substance
has been added. Do not use for intravascular injection without making
approximately isotonic by addition of suitable solute.
Discard unused portion.Rx Only
0754013
Single-dose container
Nonpyrogenic
- PRINCIPAL DISPLAY PANEL - Kit Carton - 1300 IU
- PRINCIPAL DISPLAY PANEL - 1300 IU Vial Label
-
PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
10 mL
NDC 64764-516-10Takeda
Sterile Water for Injection, USP
for reconstitution of accompanying productDo not use unless clear. No antimicrobial agent or other substance
has been added. Do not use for intravascular injection without making
approximately isotonic by addition of suitable solute.
Discard unused portion.Rx Only
0754448
Single-dose container
Nonpyrogenic
-
INGREDIENTS AND APPEARANCE
VONVENDI
von willebrand factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-7551 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-7551-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 5 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 VONVENDI
von willebrand factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0944-7550 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VON WILLEBRAND FACTOR HUMAN (UNII: ZE22NE22F1) (VON WILLEBRAND FACTOR HUMAN - UNII:ZE22NE22F1) VON WILLEBRAND FACTOR HUMAN 650 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) GLYCINE (UNII: TE7660XO1C) MANNITOL (UNII: 3OWL53L36A) TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-7550-01 5 mL in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125577 12/08/2015 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC:64764-515 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-515-50 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125577 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125577 12/08/2015 VONVENDI
von willebrand factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-7553 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-7553-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-DOSE 10 mL Part 2 1 VIAL, GLASS 10 mL Part 1 of 2 VONVENDI
von willebrand factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0944-7552 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VON WILLEBRAND FACTOR HUMAN (UNII: ZE22NE22F1) (VON WILLEBRAND FACTOR HUMAN - UNII:ZE22NE22F1) VON WILLEBRAND FACTOR HUMAN 1300 [iU] in 10 mL Inactive Ingredients Ingredient Name Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) GLYCINE (UNII: TE7660XO1C) MANNITOL (UNII: 3OWL53L36A) TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-7552-01 10 mL in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125577 12/08/2015 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC:64764-516 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 10 mL in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-516-10 10 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125577 12/08/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125577 12/08/2015 Labeler - Takeda Pharmaceuticals America, Inc. (039997266) Establishment Name Address ID/FEI Business Operations Baxalta US Inc. 009471603 MANUFACTURE(0944-7551, 0944-7553, 0944-7550, 0944-7552) , ANALYSIS(0944-7551, 0944-7553, 0944-7550, 0944-7552) , LABEL(0944-7552, 0944-7550) , PACK(0944-7553, 0944-7551) Establishment Name Address ID/FEI Business Operations Siegfried Hameln GmbH 315869123 MANUFACTURE(64764-515, 64764-516) , ANALYSIS(64764-515, 64764-516) , LABEL(64764-515, 64764-516) Establishment Name Address ID/FEI Business Operations Baxalta Manufacturing Sàrl 482124943 API MANUFACTURE(0944-7550, 0944-7552) , ANALYSIS(0944-7550, 0944-7552)