ALLEGRA COOLING RELIEF ANTI-ITCH- diphenhydramine hcl, allantoin cream
ALLEGRA INTENSIVE RELIEF ANTI-ITCH- diphenhydramine hcl, allantoin cream
Chattem, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts- Cooling Relief
Active ingredients
Allantoin 0.5%
Active ingredients
Diphenhydramine hydrochloride 2%
Purpose
Topical analgesic
Uses
- temporarily relieves pain and itching associated with:
▪ insect bites
▪ minor skin irritations
▪ sunburn
▪ rashes due to poison ivy, poison oak or poison sumac
▪ minor burns
▪ minor cuts
▪ scrapes
- temporarily protects and helps relieve chapped or cracked skin
Warnings
For external use only
Do not use on
- more than directed
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
- on deep or puncture wounds
- on animal bites
- on serious burns
- on serious sunburn or broken, blistered or oozing skin
Ask a doctor before use
- on chicken pox
- on measles
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding,
Ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- do not use more than directed
-
adults and children 2 years and older: apply freely to affected area as needed but no more than 3 or 4 times daily
-
children under 2 years: ask a doctor
Inactive ingredients
water, glycerin, petrolatum, ethanol, dimethicone, jojoba esters, menthyl lactate, cetyl alcohol, emulsifying wax, modified phospholipid, aloe vera leaf juice, stearyl alcohol, distearyldimonium chloride, poloxamer 407, steareth-21, steareth-2, glyceryl stearate, hydrolyzed jojoba esters, methyl gluceth-20, phenoxyethanol, methylparaben, tocopheryl acetate, propylparaben, EDTA, potassium hydroxide, magnesium ascorbyl phosphate, retinyl palmitate (304-79)
Drug Facts- Intensive Relief
Active ingredients
Allantoin 0.5%
Active ingredients
Diphenhydramine hydrochloride 2%
Purpose
Topical analgesic
Uses
- temporarily relieves pain and itching associated with:
▪ insect bites
▪ minor skin irritations
▪ sunburn
▪ rashes due to poison ivy, poison oak or poison sumac
▪ minor burns
▪ minor cuts
▪ scrapes
- temporarily protects and helps relieve chapped or cracked skin
Warnings
For external use only
Do not use on
- more than directed
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
- on deep or puncture wounds
- on animal bites
- on serious burns
- on serious sunburn or broken, blistered or oozing skin
Ask a doctor before use
- on chicken pox
- on measles
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding,
Ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- do not use more than directed
-
adults and children 2 years and older: apply freely to affected area as needed but no more than 3 or 4 times daily
-
children under 2 years: ask a doctor
Inactive ingredients
water, glycerin, petrolatum, ethanol, dimethicone, jojoba esters, menthyl lactate, cetyl alcohol, emulsifying wax, modified phospholipid, aloe vera leaf juice, stearyl alcohol, distearyldimonium chloride, poloxamer 407, steareth-21, steareth-2, glyceryl stearate, hydrolyzed jojoba esters, methyl gluceth-20, phenoxyethanol, methylparaben, tocopheryl acetate, propylparaben, EDTA, potassium hydroxide, magnesium ascorbyl phosphate, retinyl palmitate (304-77)
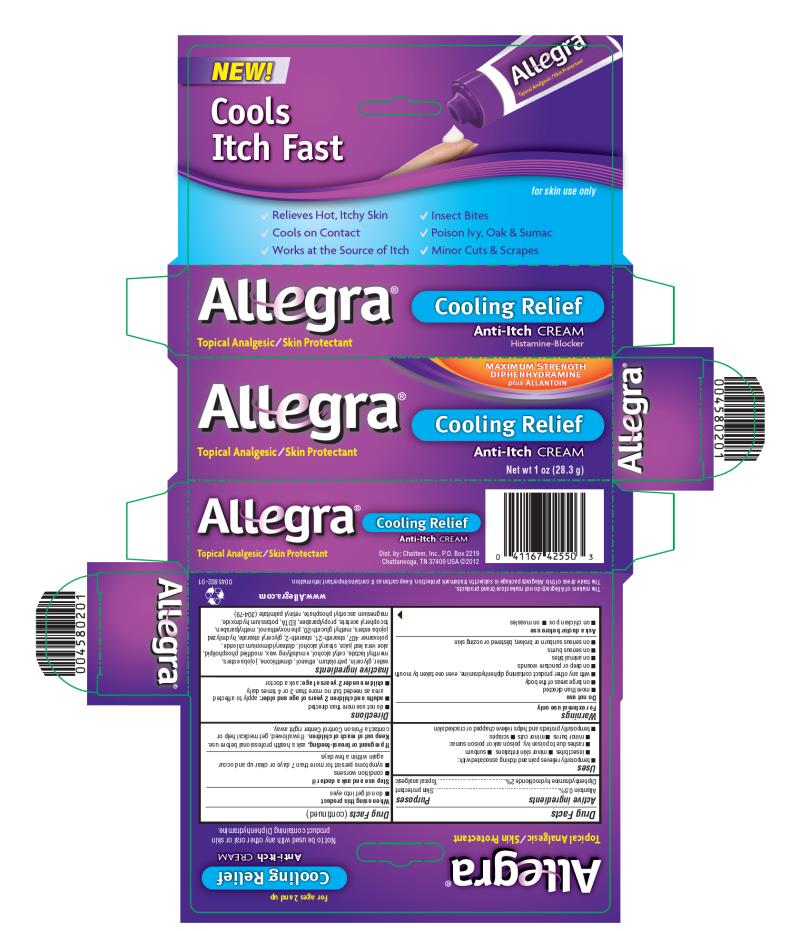
PRINCIPAL DISPLAY PANEL
NEW!
Cools Itch Fast
for skin use only
MAXIMUM STENGTH
DIPHENHYDRAMINE
plus ALLANTOIN
Cooling Relief
Anti-Itch CREAM
Histamine-Blocker
Topical Analgesic/Skin Protectant
Net wt 1 oz (28.3 g)
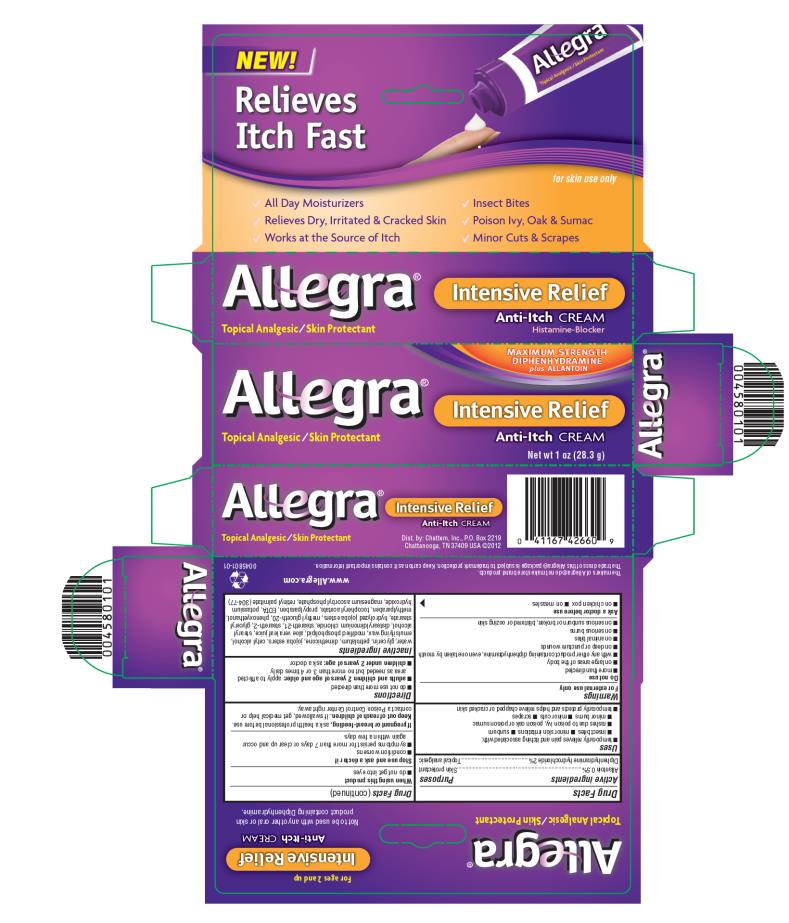
PRINCIPAL DISPLAY PANEL
NEW!
Relieves Itch Fast
for skin use only
Allegra®
MAXIMUM STENGTH
DIPHENHYDRAMINE
plus ALLANTOIN
Intensive Relief
Anti-Itch CREAM
Histamine-Blocker
Topical Analgesic/Skin Protectant
Net wt 1 oz (28.3 g)
Chattem, Inc.

