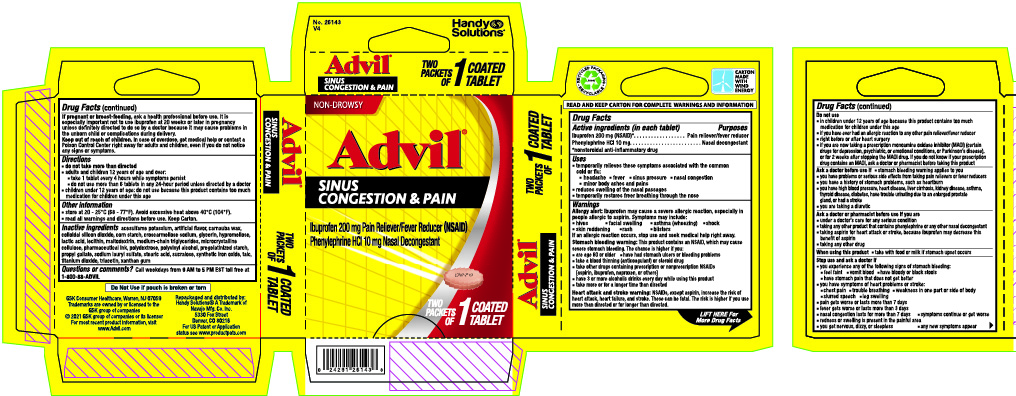
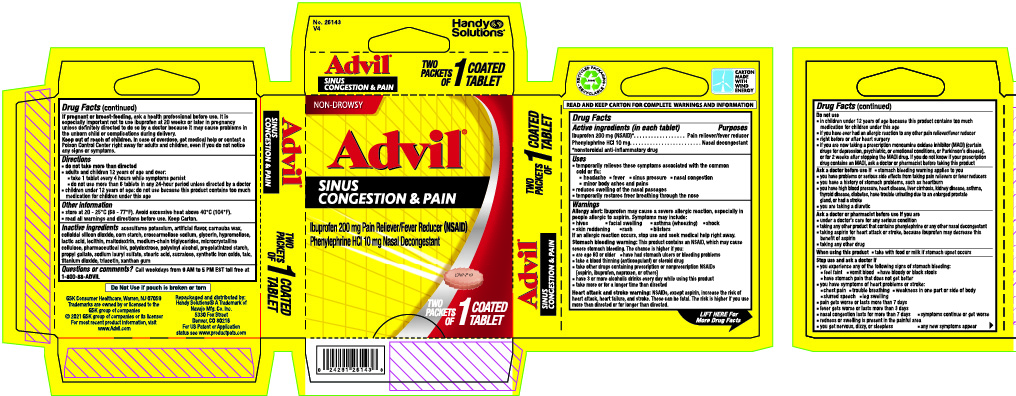
Label: ADVIL SINUS CONGESTION AND PAIN RELIEF- ibuprofen, phenylephrine hydrochloride tablet, coated
- NDC Code(s): 67751-150-01, 67751-150-02
- Packager: Navajo Manufacturing Company Inc.
- This is a repackaged label.
- Source NDC Code(s): 0573-0199
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 15, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each tablet)
- Uses
-
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
• hives • facial swelling • asthma (wheezing) • shock • rash • skin reddening • blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
• are age 60 or older • have had stomach ulcers or bleeding problems
• take a blood thinning (anticoagulant) or steroid drug
• take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
• have 3 or more alcoholic drinks every day while using this product
• take more or for a longer time than directedDo not use
• in children under 12 years of age because this product contains too much medication for children under this age
• if you have ever had an allergic reaction to any other pain reliever/fever reducer
• right before or after heart surgery
• if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this productAsk a doctor before use if
• stomach bleeding warning applies to you
• you have problems or serious side effects from taking pain relievers or fever reducers
• you have a history of stomach problems, such as heartburn
• you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, thyroid disease, diabetes or have trouble urinating due to an enlarged prostate gland
• you are taking a diureticAsk a doctor or pharmacist before use if you are
• under a doctor's care for any serious condition
• taking any other product that contains phenylephrine or any other nasal decongestant
• taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
• taking any other drugWhen using this product
• take with food or milk if stomach upset occurs
• the risk of heart attack or stroke may increase if you use more than directed or for longer than directedStop use and ask a doctor if
• you experience any of the following signs of stomach bleeding:
• feel faint • vomit blood • have bloody or black stools • have stomach pain that does not get better• pain gets worse or lasts more than 7 days
• fever gets worse or lasts more than 3 days
• nasal congestion lasts for more than 7 days
• symptoms continue or get worse
• redness or swelling is present in the painful area
• you get nervous, dizzy, or sleepless
• any new symptoms appear -
Directions
• do not take more than directed
• adults and children 12 years of age and over:
• take 1 tablet every 4 hours while symptoms persist
• do not use more than 6 tablets in any 24-hour period unless directed by a doctor
• children under 12 years of age: do not use because this product contains too much medication for children under this age - Other information
-
Inactive ingredients
acesulfame potassium, artificial flavor, carnauba wax, colloidal silicon dioxide, corn starch, croscarmellose sodium, glycerin, hypromellose, lactic acid, lecithin, maltodextrin, medium-chain triglycerides, microcrystalline cellulose, pharmaceutical ink, polydextrose, polyvinyl alcohol, pregelatinized starch, propyl gallate, sodium lauryl sulfate, stearic acid, sucralose, synthetic iron oxide, talc, titanium dioxide, triacetin, xanthan gum
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ADVIL SINUS CONGESTION AND PAIN RELIEF
ibuprofen, phenylephrine hydrochloride tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67751-150(NDC:0573-0199) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTIC ACID (UNII: 33X04XA5AT) MALTODEXTRIN (UNII: 7CVR7L4A2D) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) PROPYL GALLATE (UNII: 8D4SNN7V92) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color brown (tan) Score no score Shape OVAL Size 15mm Flavor Imprint Code 1200;P10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67751-150-01 1 in 1 CARTON 09/20/2016 1 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:67751-150-02 1 in 1 CARTON 09/20/2016 2 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022565 09/20/2016 Labeler - Navajo Manufacturing Company Inc. (091917799) Establishment Name Address ID/FEI Business Operations Navajo Manufacturing Company Inc. 136941411 relabel(67751-150) , repack(67751-150)