Label: ULTRA PROTECTION STICK BROAD SPECTRUM SPF 40 LA PRAIRIE SWITZERLAND- avobenzone, homosalate, octisalate, oxybenzone stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 68807-323-11 - Packager: Temmentec Ag
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 25, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
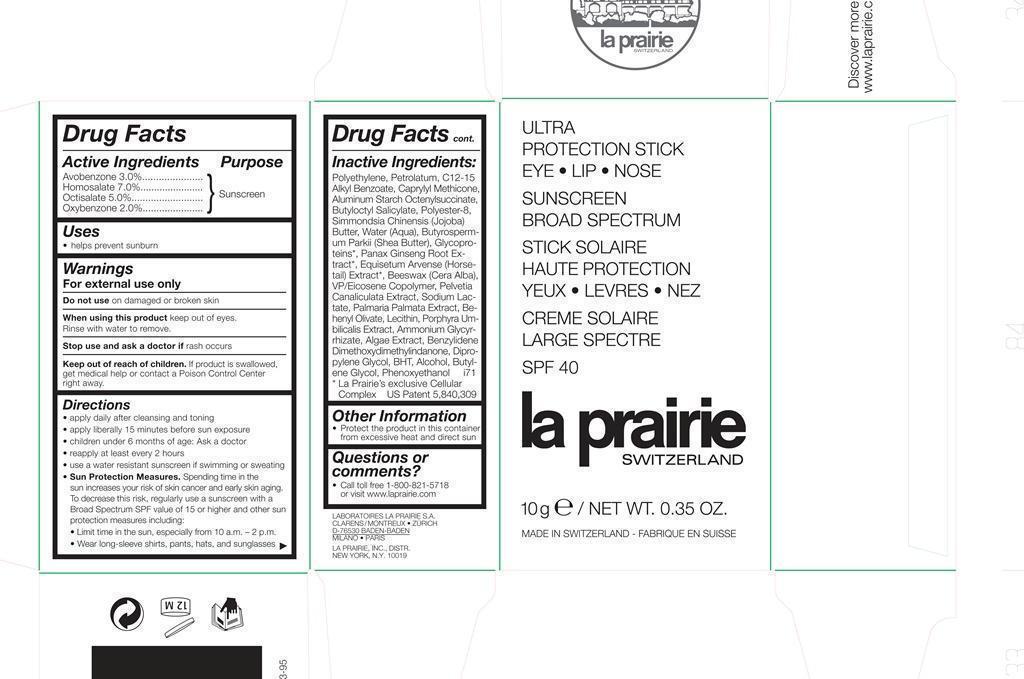
Active Ingredients Purpose
Avobenzone 3% Sunscreen
Homosalate 7% Sunscreen
Octisalate 5% Sunscreen
Oxybenzone 2% Sunscreen
Uses
helps prevent sunburn
warning
Keep out of reach of children
Indications
Stop use and ask a doctor if rash occurs
warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes.
Rinse with water to remove.
Directions
apply daily after cleansing and toning
apply liberally 15 minutes befor e sun exposure
children under 6 months of age: Ask a doctor
reapply at least every 2 hours
use a water resistant sunscreen if swimming or sweating
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun
protection measures including:
Limit time in the sun, especially from 10 a.m. – 2 p.m.
Wear long-sleeve shirts, pants, hats, and sunglasses
Inactive Ingredients Polyethylene, Petrolatum, C12-15 Alkyl Benzoate, Caprylyl Methicone, Aluminum Starch Octenylsuccinate, Butyloctyl Salicylate, Polyester-8, Simmondsia Chinensis (Jojoba) Butter, Water (Aqua), ButyrospermumParkii (Shea Butter), Glycoproteins, Panax Ginseng Root Extract, Equisetum Arvense (Horsetail) Extract, Beeswax (Cera Alba), VP/Eicosene Copolymer, Pelvetia Canaliculata Extract, Sodium Lactate, Palmaria Palmata Extract, Behenyl Olivate, Lecithin, Porphyra Umbilicalis Extract, Ammonium Glycyrrhizate, Algae Extract, Benzylidene Dimethoxydimethylindanone, Dipropylene Glycol, BHT, Alcohol, Butylene Glycol, Phenoxyethanol
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ULTRA PROTECTION STICK BROAD SPECTRUM SPF 40 LA PRAIRIE SWITZERLAND
avobenzone, homosalate, octisalate, oxybenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68807-323 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 kg HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 kg OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 kg OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 2 g in 100 kg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 350 (UNII: ZBR3T82M2V) PETROLATUM (UNII: 4T6H12BN9U) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) JOJOBA BUTTER (UNII: XIA46H803R) WATER (UNII: 059QF0KO0R) SHEA BUTTER (UNII: K49155WL9Y) ASIAN GINSENG (UNII: CUQ3A77YXI) WHITE WAX (UNII: 7G1J5DA97F) PELVETIA CANALICULATA (UNII: 8U1M44KESN) SODIUM LACTATE (UNII: TU7HW0W0QT) DULSE (UNII: 7832HOY4ZQ) BEHENYL OLIVATE (UNII: NGS1GGK4GW) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PORPHYRA UMBILICALIS (UNII: 14AN0J70WO) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) BENZYLIDENE DIMETHOXYDIMETHYLINDANONE (UNII: 75HIF3C97L) DIPROPYLENE GLYCOL (UNII: E107L85C40) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68807-323-11 78.4 kg in 1 DRUM; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/04/2013 Labeler - Temmentec Ag (480586411) Registrant - Temmentec Ag (480586411) Establishment Name Address ID/FEI Business Operations Temmentec Ag 480586411 manufacture(68807-323)