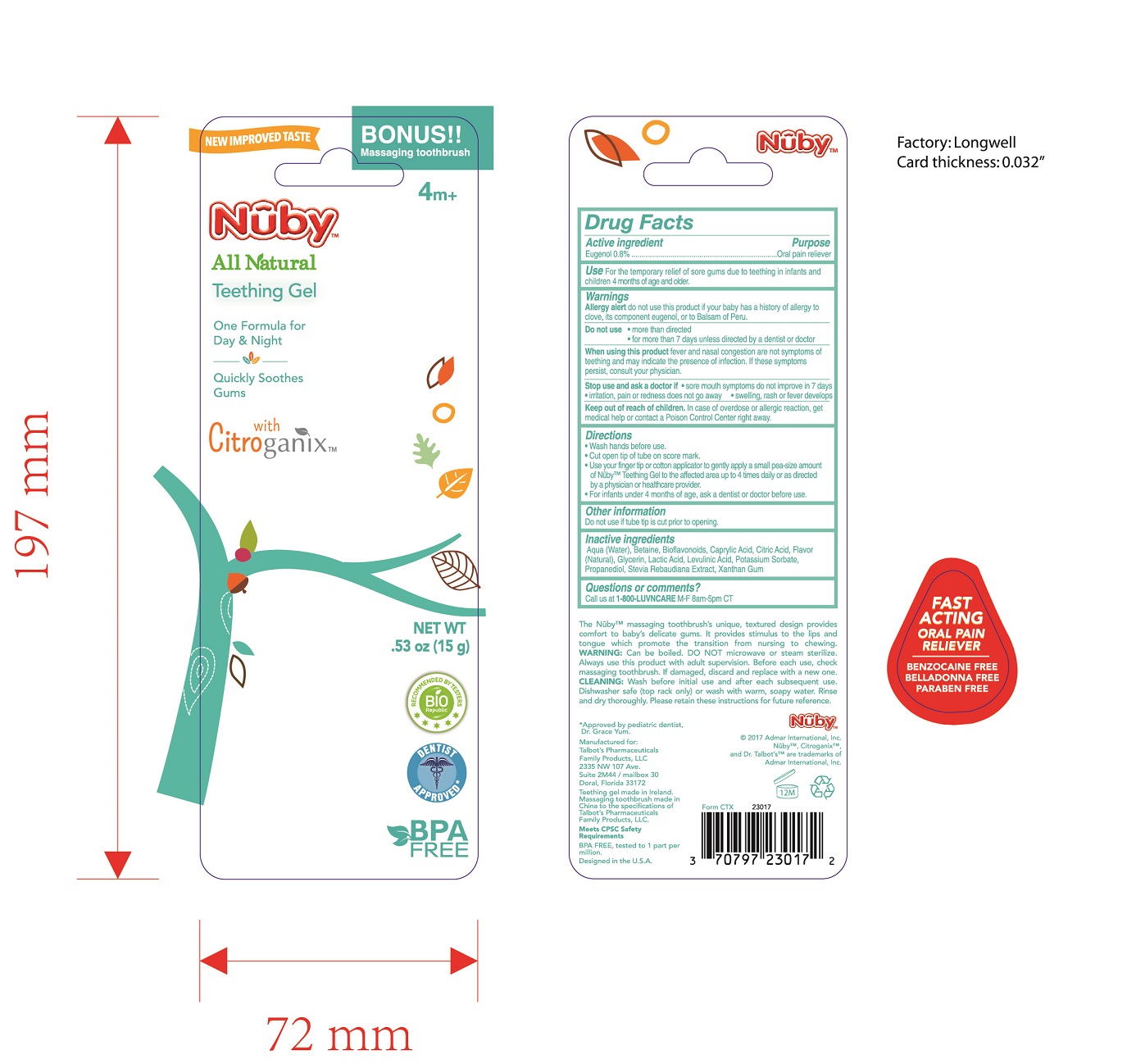
Label: NUBY TEETHING GEL- teething gel gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 71552-231-12, 71552-231-15 - Packager: Europharma Concepts limited
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 23, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Keep out of reach of children
-
Warnings
Allergy alert do not use this product if your baby has a history of allergy to clove, its component eugenol, or to Balsam of Peru.
Do not use
more than directed
for more than 7 days unless directed by a dentist or doctor
When using this product fever and nasal congestion are not symptoms of teething and may indicate the presence of infection. If these symptoms persist, consult your physician.
Stop use and ask a doctor if
sore mouth symptoms do not improve in 7 days
irritation, pain or redness does not go away
swelling, rash or fever develops
-
Directions
Wash hands before use.
Cut open tip of tube on score mark.
Use your finger tip or cotton applicator to gently apply a small pea-size amount of Nuby Teething Gel to the affected area up to 4 times daily or as directed by a physician or healthcare provider.
For infants under 4 months of age, ask a dentist or doctor before use.

- Inactive Ingredients
- Use
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NUBY TEETHING GEL
teething gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71552-231 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUGENOL (UNII: 3T8H1794QW) (EUGENOL - UNII:3T8H1794QW) EUGENOL 8 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71552-231-12 12 in 1 CARTON 06/28/2017 1 3 in 1 CARTON 1 1 in 1 BLISTER PACK 1 NDC:71552-231-15 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/28/2017 Labeler - Europharma Concepts limited (896333478)