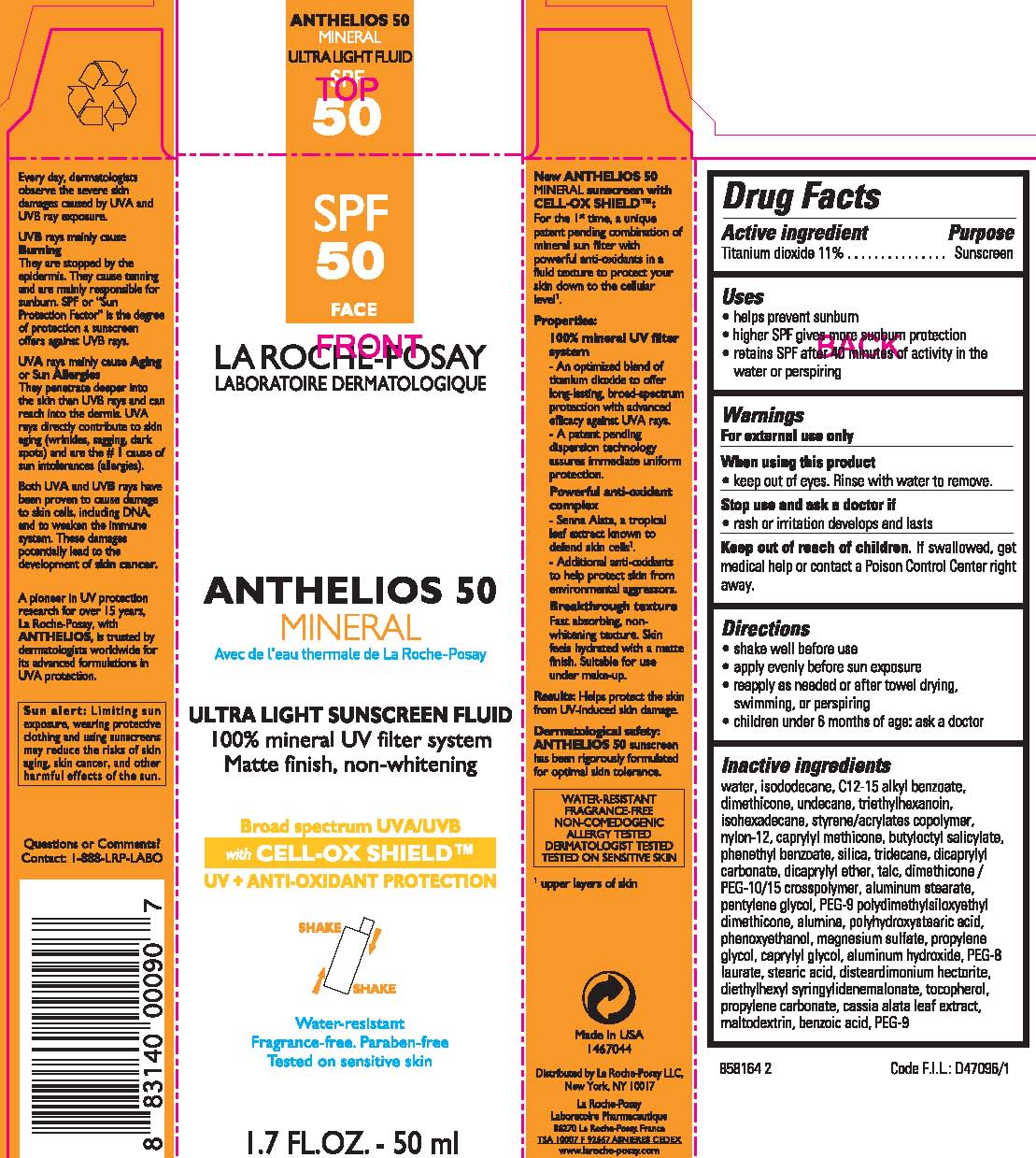
LA ROCHEPOSAY LABORATOIRE DERMATOLOGIQUE ANTHELIOS 50 MINERAL ULTRA LIGHT SUNSCREEN- titanium dioxide lotion
L'Oreal USA Products Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Titanium dioxide 11%
Warnings
For external use only
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
rash or irritation develops and lasts
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- shake well before use
- apply evenly before sun exposure
- reapply as needed or after towel drying, swimming, or perspiring
- children under 6 months of age: ask a doctor
Inactive ingredients
water, isododecane, C12-15 alkyl benzoate, dimethicone, undecane, triethylhexanoin, isohexadecane, styrene/acrylates copolymer, nylon-12, caprylyl methicone, butyloctyl salicylate, phenethyl benzoate, silica, tridecane, dicaprylyl carbonate, dicaprylyl ether, talc, dimethicone/PEG-10/15 crosspolymer, aluminum stearate, pentylene glycol, PEG-9 polydimethylsiloxyethyl dimethicone, alumina, polyhydroxystearic acid, phenoxyethanol, magnesium sulfate, protpylene glycol, caprylyl glycol, aluminum hydroxide, PEG-8 laurate, stearic acid, disteardimonium hectorite, diethylhexyl syringylidenemalonate, tocopherol, propylene carbonate, cassia alata leaf extract, maltodextrin, benzoic acid, PEG-9
Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
- retains SPF after 40 minutes of activity in the water or perspiring