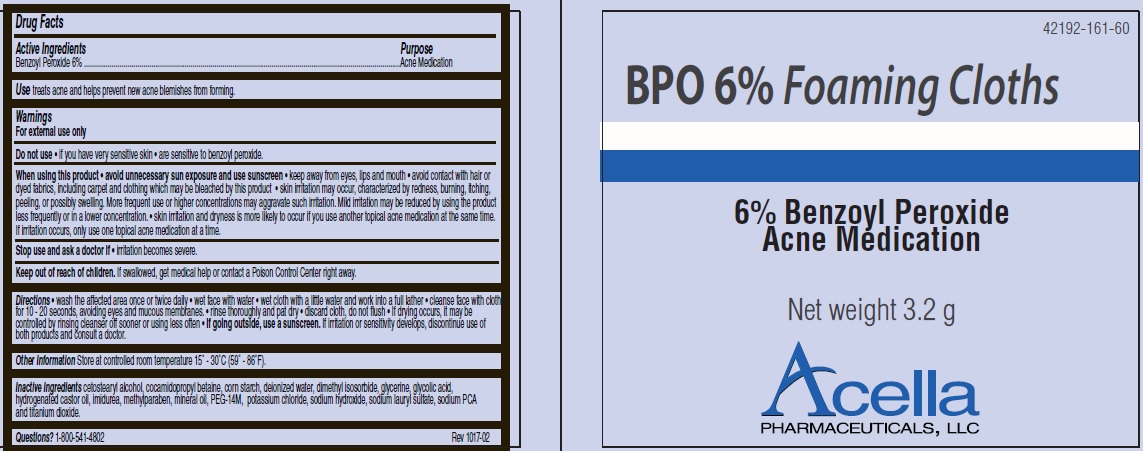
BPO 6%- benzoyl peroxide cloth
Acella Pharmaceuticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BPO 6% Foaming Cloths
Warnings
For external use only
When using this product
- avoid unnecessary sun exposure and use sunscreen
- keep away from eyes, lips and mouth
- avoid contact with hair or dyed fabrics, including carpet and clothing which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. More frequent use or higher concentrations may aggravate such irritation. Mild irritation may be reduced by using the product less frequently or in a lower concentration.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Directions
- wash the affected area once or twice daily
- wet face with water
- wet cloth with a little water and work into a full lather
- cleanse face with cloth for 10 - 20 seconds, avoiding eyes and mucous membranes.
- rinse thoroughly and pat dry
- discard cloth, do not flush
- If drying occurs, it may be controlled by rinsing cleanser off sooner or using less often
- If going outside, use a sunscreen. If irritation or sensitivity develops, discontinue use of both products and consult a doctor.
Inactive Ingredients
cetostearyl alcohol, cocamidopropyl betaine, corn starch, deionized water, dimethyl isosorbide, glycerine, glycolic acid, hydrogenated castor oil, imidurea, methylparaben, mineral oil, PEG-14M, potassium chloride, sodium hydroxide, sodium lauryl sulfate, sodium PCA and titanium dioxide.
Questions? 1-800-541-4802
Manufactured by:
Acella Pharmaceuticals, LLC
1-800-541-4802
Rev 1017-02
| BPO 6%
benzoyl peroxide cloth |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Acella Pharmaceuticals, LLC (825380939) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Acella Pharmaceuticals, LLC | 825380939 | MANUFACTURE(42192-161) | |
Revised: 9/2023
Document Id: 61b7b789-0e10-4dd4-a2ce-cdb08916203c
Set id: 52b3826a-3b3d-4a13-9d18-4ff8882cc2bf
Version: 3
Effective Time: 20230920
Acella Pharmaceuticals, LLC