COLGATE TOTAL ADVANCED FRESH PLUS WHITENING- sodium fluoride and triclosan paste, dentifrice
Colgate-Palmolive Company
----------
Colgate® Total® Advanced Fresh plus Whitening
Warnings
Directions
supervise children as necessary until capable of using without supervision.
| adults and children 6 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or a physician |
| children under 12 years | instruct in good brushing and rinsing habits (to minimize swallowing) |
| children under 6 years | do not use unless directed by a dentist or a physician |
Antiplaque and antigingivitis use not proven in children.
Inactive ingredients
Water, hydrated silica, glycerin, sorbitol, flavor, PVM/MA copolymer, sodium lauryl sulfate, cellulose gum, sodium hydroxide, propylene glycol, carrageenan, sodium saccharin, mica, titanium dioxide, FD&C blue no. 1, D&C yellow no. 10
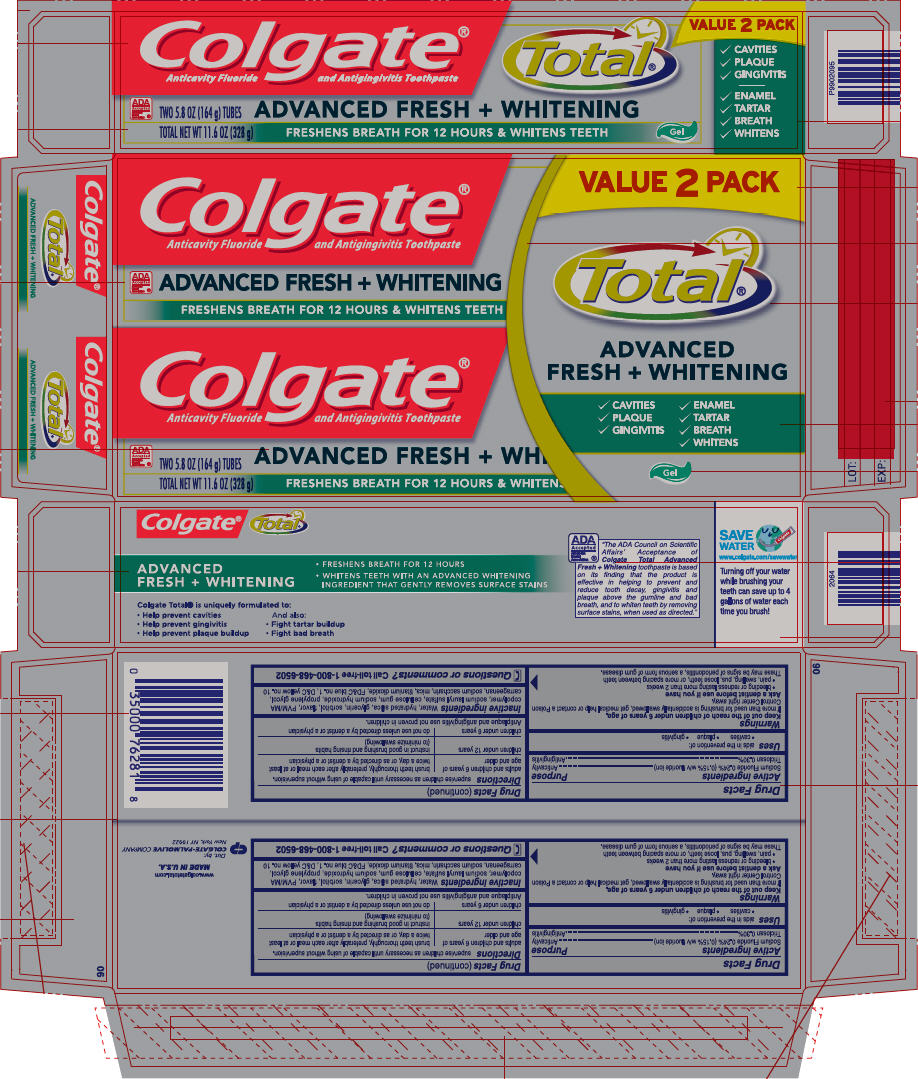
PRINCIPAL DISPLAY PANEL - 164 g Tube Carton
Colgate®Total®
Anticavity Fluoride and Antigingivitis Toothpaste
VALUE 2 PACK
✓ CAVITIES
✓ PLAQUE
✓ GINGIVITIS
✓ ENAMEL
✓ TARTAR
✓ BREATH
✓ WHITENS
ADA
Accepted
American
Dental
Association ®
TWO 5.8 OZ (164 g) TUBES
TOTAL NET WT 11.6 OZ (328 g)
ADVANCED FRESH + WHITENING
FRESHENS BREATH FOR 12 HOURS & WHITENS TEETH
Gel
| COLGATE TOTAL ADVANCED FRESH PLUS WHITENING
sodium fluoride and triclosan paste, dentifrice |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Colgate-Palmolive Company (001344381) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Colgate-Palmolive | 785047999 | MANUFACTURE(35000-004) | |