METHENAMINE HIPPURATE- methenamine hippurate tablet
County Line Pharmaceuticals, LLC
----------
Methenamine Hippurate Tablets 1 g
PI -CLP020A
02/10
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Methenamine Hippurate Tablets and other antibacterial drugs, Methenamine Hippurate Tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Methenamine Hippurate Tablets are a urinary tract antiseptic drug. Each white, scored tablet contains methenamine hippurate 1g (see HOW SUPPLIED). Methenamine Hippurate Tablets also contain: magnesium stearate, povidone and saccharin sodium. Chemically, methenamine hippurate is the hippuric acid salt of methenamine (hexamethylenetetramine).
Structural formula:
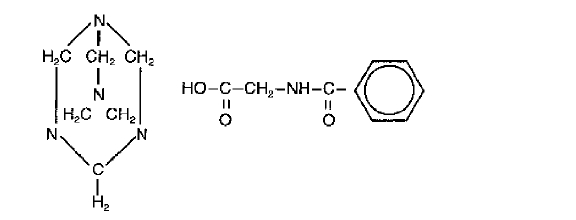
C15H21N5O3
Molecular Weight 319.37
CLINICAL PHARMACOLOGY
Methenamine hippurate is readily absorbed from the GI tract. Methenamine distributes widely into body fluids, but very little is hydrolyzed prior to excretion in the kidney and thus has minimal systemic toxic potential.
Within one-half hour after a single 1 g dose of a Methenamine Hippurate Tablet, antibacterial activity is demonstrable in the urine. Urine shows continuous antibacterial activity when Methenamine Hippurate Tablets are administered at the recommended dosage schedule of 1 g twice daily. Over 90% of the methenamine moiety is excreted in the urine within twenty-four hours after administration of a single 1 g dose. Similarly, the hippurate moiety is rapidly absorbed and excreted, and it reaches the urine by both tubular secretion and glomerular filtration. This may be of importance in older patients or those with some degree of renal impairment.
Methenamine is placentally transferred to the fetus during pregnancy.
Microbiology: Methenamine hippurate exerts its activity because the methenamine component is hydrolyzed to formaldehyde in acid urine. Hippuric acid, the other component, acts to keep the urine acid. The minimal inhibitory concentrations are significantly lower in more acidic media; therefore, the efficacy of Methenamine Hippurate Tablets can be increased by acidification of urine (see DOSAGE AND ADMINISTRATION).
Microorganisms do not develop resistance to formaldehyde; however urea-splitting microorganisms (e.g. Proteus species) tend to raise pH of the urine thus inhibiting the release of formaldehyde. When the urine pH is 6 and the daily urine volume is 1000 -1500 mL a 2 g dose of Methenamine Hippurate Tablets daily will yield a urinary concentration of 18 to 60 μg/mL of formaldehyde, this being more than the minimal inhibitory concentration for most urinary pathogens.
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Methenamine Hippurate Tablets and other antibacterial drugs, Methenamine Hippurate Tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Methenamine Hippurate Tablets are indicated for prophylactic or suppressive treatment of frequently recurring urinary tract infections when long-term therapy is considered necessary. This drug should only be used after eradication of the infection by other appropriate antimicrobial agents.
CONTRAINDICATIONS
Methenamine Hippurate Tablets are contraindicated in patients with renal insufficiency, severe hepatic insufficiency, or severe dehydration. It should not be used as the sole therapeutic agent in acute parenchymal infections causing systemic symptoms.
WARNINGS
Patients with pre-existing hepatic insufficiency may suffer adverse effects from the small amounts of ammonia and formaldehyde that are produced. The classical syndrome of acute hepatic failure may be evoked in these patients.
PRECAUTIONS
General:
Prescribing Methenamine Hippurate Tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Large doses of methenamine (8 g daily for 3 to 4 weeks) have caused bladder irritation, painful and frequent micturition, albuminuria and gross hematuria.
Care should be taken to maintain an acid pH of the urine especially when treating infections due to urea-splitting organisms such as Proteus spp. and strains of Pseudomonas spp.
Information for Patients:
Patients should be counseled that antibacterial drugs including Methenamine Hippurate Tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g. the common cold). When Methenamine Hippurate Tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Methenamine Hippurate Tablets or other antibacterial drugs in the future.
Laboratory Tests:
In a few instances in one study, the serum transaminase levels showed a mild elevation during treatment which returned to normal while the patients were still receiving Methenamine Hippurate Tablets. Because of this one report, it is recommended that liver function studies be performed periodically on patients receiving the drug, especially those with liver dysfunction.
Drug Interactions:
The concomitant administration of methenamine hippurate and sulfamethizole or sulfathiazole is liable to result in the formation of a precipitate in the urine.
Drug/Laboratory Test Interactions:
Methenamine causes spuriously elevated urinary 17-hydroxycorticosteroid and catecholamine levels.
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Methenamine Hippurate Tablets have not been evaluated for carcinogenicity or mutagenicity.
Methenamine was evaluated for mutagenicity in the Ames Salmonella/mammalian microsome test. Five strains of Salmonella typhimurium (TA98, TA100, TA1535, TA1537 and TA1538) and a strain of Escherichia coli (WP2uvrA) were used. At doses of 10,000 μg/plate methenamine showed mutagenic activity in Salmonella typhimurium TA98 and TA100 by metabolic activation and also showed mutagenic activity in TA98 without microsomal activation.
In one large study, no evidence of carcinogenicity was found following long-term oral administration of methenamine 1.25g/kg/day to rats (104 weeks) and mice (60 weeks). The same investigators also reported no suggestion of carcinogenicity resulting from five subcutaneous injections of 5 g/kg (given on alternate days for a total dose of 25 g/kg). An earlier, much smaller study showed a 50% incidence of local sarcomas following subcutaneous injection of methenamine, totaling 25 g/kg, administered over periods of up to 15 months to rats concurrently receiving formic acid.
Methenamine hippurate administered at a dose level of 800 mg/kg/day, did not adversely affect the fertility of female rats. Effects on male fertility have not been adequately studied.
Pregnancy:
Teratogenic effects. Pregnancy category C. Oral administration of methenamine to pregnant dogs, at doses equivalent to the human dose, has been reported to cause a slight increase in the stillborn rate and slight impairment of weight gain and survival of live-born offspring. A teratogenicity study, in which methenamine hippurate was administered to pregnant rabbits at doses approximately 3 times human dose, revealed no evidence of harm to the fetus. There are no adequate and well-controlled studies in pregnant women. Methenamine Hippurate Tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery:
Methenamine Hippurate Tablets have no recognized use during labor and delivery, and its effect during these processes is unknown.
ADVERSE REACTIONS
Adverse effects of Methenamine Hippurate Tablets have been reported in fewer than 3.5% of patients treated.
These reactions have included the following, in decreasing order of frequency: nausea, vomiting and rarely pruritus, rash, dysuria.
Children have received Methenamine Hippurate Tablets at the recommended dosages as a prophylactic/suppressive regimen after initial treatment of acute episodes of pyuria. Side effects were encountered in only 1.1% of these children.
OVERDOSAGE
Immediately after ingestion of an overdose, further absorption of the drug may be minimized by inducing vomiting or by gastric lavage, followed by administration of activated charcoal. Fluids, either oral or parenteral, should be forced to tolerance.
DOSAGE AND ADMINISTRATION
One tablet (1 g) twice daily for adults and children over 12 years of age. One-half tablet or one tablet (0.5 or 1 g) twice daily for children 6 to 12 years of age.
The antibacterial activity of Methenamine Hippurate Tablets is greater in acid urine. Therefore, restriction of alkalinizing foods and medications is desirable. If necessary, as indicated by urinary pH and clinical response, supplemental acidification of the urine may be instituted. The efficacy of therapy should be monitored by repeated urine cultures.
HOW SUPPLIED
Methenamine Hippurate Tablets are capsule-shaped, scored, white, imprinted “VP” on one side and “UREX” on the other. Each tablet contains methenamine hippurate 1 g.
Bottles of 100 tablets (NDC 43199-020-01).
Store at controlled room temperature 15°-30°C (59°-86°F).
ANIMAL PHARMACOLOGY AND ANIMAL TOXICOLOGY
Up to 600 mg/kg of methenamine hippurate in a single dose have been administered intravenously to dogs and to rats without toxic effects being observed. Chronic oral administration of 50 to 200 mg/kg/day to dogs and 800 to 6400 mg/kg/day to rats produced gastric and bladder irritation with some hemorrhagic sites and ulcerations observed at autopsy. Amounts of methenamine hippurate equivalent to twice the recommended human dose were administered to rats for twelve months and to monkeys for six months without producing any adverse effects.
Rx only
Distributed by:
County Line Pharmaceuticals, LLC
Brookfield, WI 53005
For Inquires Call:
1-866-207-5636
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

NDC 43199-020-01
100 Tablets
Methenamine Hippurate
Tablets 1g
Rx only
Each tablet contains: Methenamine hippurate 1g.
Dosage: Adults: one tablet twice daily.
Children: over 12 years of age, one tablet twice daily; 6 to 12 years of age, one-half or one tablet twice daily. Consult accompanying professional literature.
Pharmacists: This is a bulk package. Dispense in a tight, light-resistant container. Store below 30° C (86° F).
Manufactured For: County Line Pharmaceuticals, LLC
Brookfield, WI 53005
| METHENAMINE HIPPURATE
methenamine hippurate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - County Line Pharmaceuticals, LLC (015585278) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ANI Pharmaceuticals, Inc. | 148515737 | manufacture(43199-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ANI Pharmaceuticals, Inc. | 831049809 | manufacture(43199-020) | |