DIVALPROEX SODIUM- divalproex sodium tablet, film coated, extended release
REMEDYREPACK INC.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Divalproex sodium extended release tablets safely and effectively.See full prescribing information for divalproex sodium extended release tablets. Divalproex Sodium Extended Release Tablets for Oral use. Initial U.S. approval:2000
WARNING: LIFE THREATENING ADVERSE REACTIONS
|
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNING: LIFE THREATENING ADVERSE REACTIONS
Hepatotoxicity
Hepatic failure resulting in fatalities has occurred in patients receiving valproic acid and its derivatives. Children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those on multiple anticonvulsants, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease. When divalproex sodium extended-release tablets are used in this patient group, it should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. The incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Liver function tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months [see Warnings and Precautions (5.1)].
Teratogenicity
Valproate can produce teratogenic effects such as neural tube defects (e.g., spina bifida). Accordingly, the use of divalproex sodium extended-release tablets in women of childbearing potential requires that the benefits of its use be weighed against the risk of injury to the fetus. This is especially important when the treatment of a spontaneously reversible condition not ordinarily associated with permanent injury or risk of death (e.g., migraine) is contemplated [see Warnings and Precautions (5.2)].
An information sheet describing the teratogenic potential of valproate is available for patients [see Patient Counseling Information (17.8)].
Pancreatitis
Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with a rapid progression from initial symptoms to death. Cases have been reported shortly after initial use as well as after several years of use. Patients and guardians should be warned that abdominal pain, nausea, vomiting and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, valproate should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated [see Warnings and Precautions (5.3)].
1INDICATIONS AND USAGE
1.1Mania
Divalproex sodium extended-release tablet is a valproate and is indicated for the treatment of acute manic or mixed episodes associated with bipolar disorder, with or without psychotic features. A manic episode is a distinct period of abnormally and persistently elevated, expansive, or irritable mood. Typical symptoms of mania include pressure of speech, motor hyperactivity, reduced need for sleep, flight of ideas, grandiosity, poor judgment, aggressiveness, and possible hostility. A mixed episode is characterized by the criteria for a manic episode in conjunction with those for a major depressive episode (depressed mood, loss of interest or pleasure in nearly all activities).
The efficacy of divalproex sodium extended-release tablet is based in part on studies of divalproex sodium (delayed release tablets) in this indication, and was confirmed in a 3-week trial with patients meeting DSM-IV TR criteria for bipolar I disorder, manic or mixed type, who were hospitalized for acute mania [see Clinical Studies (14.1)].
The safety and effectiveness of valproate for long-term use in mania, i.e., more than 3 weeks, has not been systematically evaluated in controlled clinical trials. Therefore, healthcare providers who elect to use divalproex sodium extended-release tablets for extended periods should continually reevaluate the long-term risk-benefits of the drug for the individual patient.
1.2Epilepsy
Divalproex sodium extended-release tablets are indicated as monotherapy and adjunctive therapy in the treatment of adult patients and pediatric patients down to the age of 10 years with complex partial seizures that occur either in isolation or in association with other types of seizures. Divalproex sodium extended-release tablets are also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures in adults and children 10 years of age or older, and adjunctively in adults and children 10 years of age or older with multiple seizure types that include absence seizures.
Simple absence is defined as very brief clouding of the sensorium or loss of consciousness accompanied by certain generalized epileptic discharges without other detectable clinical signs. Complex absence is the term used when other signs are also present.
1.3Migraine
Divalproex sodium extended-release tablets are indicated for prophylaxis of migraine headaches. There is no evidence that divalproex sodium extended-release tablets are useful in the acute treatment of migraine headaches. Because it may be a hazard to the fetus, divalproex sodium extended-release tablets should be considered for women of childbearing potential only after this risk has been thoroughly discussed with the patient and weighed against the potential benefits of treatment [see Warnings and Precautions (5.2), Patient Counseling Information (17.3)].
2DOSAGE AND ADMINISTRATION
Divalproex sodium extended-release tablets are intended for once-a-day oral administration. Divalproex sodium extended-release tablets should be swallowed whole and should not be crushed or chewed.
2.1Mania
Divalproex sodium extended-release tablets are administered orally. The recommended initial dose is 25 mg/kg/day given once daily. The dose should be increased as rapidly as possible to achieve the lowest therapeutic dose which produces the desired clinical effect or the desired range of plasma concentrations. In a placebo-controlled clinical trial of acute mania or mixed type, patients were dosed to a clinical response with a trough plasma concentration between 85 and 125 mcg/mL. The maximum recommended dosage is 60 mg/kg/day.
There is no body of evidence available from controlled trials to guide a clinician in the longer term management of a patient who improves during divalproex sodium extended-release tablets treatment of an acute manic episode. While it is generally agreed that pharmacological treatment beyond an acute response in mania is desirable, both for maintenance of the initial response and for prevention of new manic episodes, there are no systematically obtained data to support the benefits of divalproex sodium extended-release tablets in such longer-term treatment (i.e., beyond 3 weeks).
2.2Epilepsy
Divalproex sodium extended-release tablets are administered orally, and must be swallowed whole. As divalproex sodium extended-release tablets dosage is titrated upward, concentrations of clonazepam, diazepam, ethosuximide, lamotrigine, tolbutamide, phenobarbital, carbamazepine, and/or phenytoin may be affected [see Drug Interactions (7.2)].
Complex Partial Seizures
For adults and children 10 years of age or older.
Monotherapy (Initial Therapy)
Divalproex sodium extended-release tablets have not been systematically studied as initial therapy. Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made.
The probability of thrombocytopenia increases significantly at total trough valproate plasma concentrations above 110 mcg/mL in females and 135 mcg/mL in males. The benefit of improved seizure control with higher doses should be weighed against the possibility of a greater incidence of adverse reactions.
Conversion to Monotherapy
Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 - 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made.
Concomitant antiepilepsy drug (AED) dosage can ordinarily be reduced by approximately 25% every 2 weeks. This reduction may be started at initiation of divalproex sodium extended-release tablets therapy, or delayed by 1 to 2 weeks if there is a concern that seizures are likely to occur with a reduction. The speed and duration of withdrawal of the concomitant AED can be highly variable, and patients should be monitored closely during this period for increased seizure frequency.
Adjunctive Therapy
Divalproex sodium extended-release tablets may be added to the patient's regimen at a dosage of 10 to 15 mg/kg/day. The dosage may be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day.
If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made.
In a study of adjunctive therapy for complex partial seizures in which patients were receiving either carbamazepine or phenytoin in addition to valproate, no adjustment of carbamazepine or phenytoin dosage was needed [see Clinical Studies (14.3)]. However, since valproate may interact with these or other concurrently administered AEDs as well as other drugs, periodic plasma concentration determinations of concomitant AEDs are recommended during the early course of therapy [see Drug Interactions (7)].
Simple and Complex Absence Seizures
The recommended initial dose is 15 mg/kg/day, increasing at one week intervals by 5 to 10 mg/kg/day until seizures are controlled or side effects preclude further increases. The maximum recommended dosage is 60 mg/kg/day.
A good correlation has not been established between daily dose, serum concentrations, and therapeutic effect. However, therapeutic valproate serum concentration for most patients with absence seizures is considered to range from 50 to 100 mcg/mL. Some patients may be controlled with lower or higher serum concentrations [see Clinical Pharmacology (12.3)].
As divalproex sodium extended-release tablets dosage is titrated upward, blood concentrations of phenobarbital and/or phenytoin may be affected [see Drug Interactions (7.2)].
Antiepilepsy drugs should not be abruptly discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life.
2.3Migraine
Divalproex sodium extended-release tablets are indicated for prophylaxis of migraine headaches in adults.
The recommended starting dose is 500 mg once daily for 1 week, thereafter increasing to 1000 mg once daily. Although doses other than 1000 mg once daily of divalproex sodium extended-release tablets have not been evaluated in patients with migraine, the effective dose range of divalproex sodium delayed-release tablets in these patients is 500-1000 mg/day. As with other valproate products, doses of divalproex sodium extended-release tablets should be individualized and dose adjustment may be necessary. If a patient requires smaller dose adjustments than that available with divalproex sodium extended release tablets, divalproex sodium delayed-release tablets should be used instead.
2.4Conversion from Divalproex Sodium Delayed-Release Tablets to Divalproex Sodium Extended-Release Tablets
In adult patients and pediatric patients 10 years of age or older with epilepsy previously receiving divalproex sodium delayed-release tablets, divalproex sodium extended-release tablets should be administered once-daily using a dose 8 to 20% higher than the total daily dose of divalproex sodium delayed-release tablets (Table 1). For patients whose divalproex sodium delayed-release tablets total daily dose cannot be directly converted to divalproex sodium extended-release tablets, consideration may be given at the clinician's discretion to increase the patient's divalproex sodium delayed-release tablets total daily dose to the next higher dosage before converting to the appropriate total daily dose of divalproex sodium extended-release tablets.
| Divalproex Sodium Delayed-Release Tablets Total Daily Dose (mg) | Divalproex Sodium Extended-Release Tablets (mg) |
|---|---|
| 500*-625 | 750 |
| 750*-875 | 1000 |
| 1000*-1125 | 1250 |
| 1250-1375 | 1500 |
| 1500-1625 | 1750 |
| 1750 | 2000 |
| 1875-2000 | 2250 |
| 1225-2250 | 2500 |
| 2375 | 2750 |
| 2500-2750 | 3000 |
| 2875 | 3250 |
| 3000-3125 | 3500 |
* These total daily doses of divalproex sodium delayed-release tablets cannot be directly converted to an 8 to 20% higher total daily dose of divalproex sodium extended-release tablets because the required dosing strengths of divalproex sodium extended-release tablets are not available. Consideration may be given at the clinician's discretion to increase the patient's divalproex sodium delayed-release tablets total daily dose to the next higher dosage before converting to the appropriate total daily dose of divalproex sodium extended-release tablets.
There is insufficient data to allow a conversion factor recommendation for patients with divalproex sodium delayed-release tablets doses above 3125 mg/day. Plasma valproate C
min concentrations for divalproex sodium extended-release tablets on average are equivalent to divalproex sodium delayed-release tablets, but may vary across patients after conversion. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL) [see Clinical Pharmacology (12.2)].
2.5General Dosing Advice
Dosing in Elderly Patients
Due to a decrease in unbound clearance of valproate and possibly a greater sensitivity to somnolence in the elderly, the starting dose should be reduced in these patients. Starting doses in the elderly lower than 250 mg can only be achieved by the use of divalproex sodium delayed-release tablets. Dosage should be increased more slowly and with regular monitoring for fluid and nutritional intake, dehydration, somnolence, and other adverse reactions. Dose reductions or discontinuation of valproate should be considered in patients with decreased food or fluid intake and in patients with excessive somnolence. The ultimate therapeutic dose should be achieved on the basis of both tolerability and clinical response [see Warnings and Precautions (5.12)].
Dose-Related Adverse reactions
The frequency of adverse effects (particularly elevated liver enzymes and thrombocytopenia) may be dose-related. The probability of thrombocytopenia appears to increase significantly at total valproate concentrations of
>
110 mcg/mL (females) or
>
135 mcg/mL (males) [see Warnings and Precautions (5.6)]. The benefit of improved therapeutic effect with higher doses should be weighed against the possibility of a greater incidence of adverse reactions.
G.I. Irritation
Patients who experience G.I. irritation may benefit from administration of the drug with food or by slowly building up the dose from an initial low level.
Compliance
Patients should be informed to take divalproex sodium extended-release tablets every day as prescribed. If a dose is missed it should be taken as soon as possible, unless it is almost time for the next dose. If a dose is skipped, the patient should not double the next dose.
3DOSAGE FORMS AND STRENGTHS
Divalproex sodium extended-release 250 mg tablets are available as white, oval shaped film coated biconvex beveled edge tablets, debossed with W on one side and 724 on other side. Each divalproex sodium extended-release tablet contains divalproex sodium equivalent to 250 mg of valproic acid in the following packaging sizes:
Bottles of 30 (NDC 64679-724-01)
Bottles of 100 (NDC 64679-724-02)
Bottles of 500 (NDC 64679-724-03)
Unit Dose Pack of 100 (10 x 10)(NDC 64679-724-04)
Divalproex sodium extended-release 500 mg tablets are available as dark grey colored, oval shaped film coated biconvex tablets, debossed with W725 on one side and plain on other side. Each divalproex sodium extended-release tablet contains divalproex sodium equivalent to 500 mg of valproic acid in the following packaging sizes:
Bottles of 30 (NDC 64679-725-01)
Bottles of 500 (NDC 64679-725-03)
Unit Dose Packages of 100 (10x10) (NDC 64679-725-04)
4CONTRAINDICATIONS
- Divalproex sodium extended-release tablets should not be administered to patients with hepatic disease or significant hepatic dysfunction [see Warnings and Precautions (5.1)].
- Divalproex sodium extended-release tablets are contraindicated in patients with known hypersensitivity to the drug [see Warnings and Precautions (5.10)].
- Divalproex sodium extended-release tablets are contraindicated in patients with known urea cycle disorders [see Warnings and Precautions (5.4)].
5WARNINGS AND PRECAUTIONS
5.1Hepatotoxicity
Hepatic failure resulting in fatalities has occurred in patients receiving valproic acid. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Liver function tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months. However, healthcare providers should not rely totally on serum biochemistry since these tests may not be abnormal in all instances, but should also consider the results of careful interim medical history and physical examination.
Caution should be observed when administering valproic acid products to patients with a prior history of hepatic disease.
Patients on multiple anticonvulsants, children, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease may be at particular risk. Experience has indicated that children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those with the aforementioned conditions. When divalproex sodium extended-release tablet is used in this patient group, it should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. Above this age group, experience in epilepsy has indicated that the incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
The drug should be discontinued immediately in the presence of significant hepatic dysfunction, suspected or apparent. In some cases, hepatic dysfunction has progressed in spite of discontinuation of drug [see Boxed Warning and Contraindications (4)].
5.2Teratogenicity/Usage in Pregnancy
Use of divalproex sodium extended-release tablets during pregnancy can cause congenital malformations including neural tube defects. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Divalproex sodium extended-release tablets should be considered for women of childbearing potential only after the risks have been thoroughly discussed with the patient and weighed against the potential benefits of treatment.
Data suggest that there is an increased incidence of congenital malformations associated with the use of valproate by women with seizure disorders during pregnancy when compared to the incidence in women with seizure disorders who do not use antiepileptic drugs during pregnancy, the incidence in women with seizure disorders who use other antiepileptic drugs, and the background incidence for the general population.
The data described below were gained almost exclusively from women who received valproate to treat epilepsy. There are multiple reports in the clinical literature that indicate the use of antiepileptic drugs during pregnancy results in an increased incidence of congenital malformations in offspring. Antiepileptic drugs, including valproate, should be administered to women of childbearing potential only if they are clearly shown to be essential in the management of their medical condition.
Antiepileptic drugs should not be discontinued abruptly in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that the removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy, although it cannot be said with any confidence that even minor seizures do not pose some hazard to the developing embryo or fetus. [see Boxed Warning and Use in Specific Populations(8.1)].
5.3Pancreatitis
Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with rapid progression from initial symptoms to death. Some cases have occurred shortly after initial use as well as after several years of use. The rate based upon the reported cases exceeds that expected in the general population and there have been cases in which pancreatitis recurred after rechallenge with valproate. In clinical trials, there were 2 cases of pancreatitis without alternative etiology in 2416 patients, representing 1044 patient-years experience. Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, divalproex sodium extended-release tablets should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated [see Boxed Warning].
5.4Urea Cycle Disorders
Divalproex sodium extended-release tablets are contraindicated in patients with known urea cycle disorders (UCD). Hyperammonemic encephalopathy, sometimes fatal, has been reported following initiation of valproate therapy in patients with urea cycle disorders, a group of uncommon genetic abnormalities, particularly ornithine transcarbamylase deficiency. Prior to the initiation of divalproex sodium extended-release tablets therapy, evaluation for UCD should be considered in the following patients: 1) those with a history of unexplained encephalopathy or coma, encephalopathy associated with a protein load, pregnancy-related or postpartum encephalopathy, unexplained mental retardation, or history of elevated plasma ammonia or glutamine; 2) those with cyclical vomiting and lethargy, episodic extreme irritability, ataxia, low BUN, or protein avoidance; 3) those with a family history of UCD or a family history of unexplained infant deaths (particularly males); 4) those with other signs or symptoms of UCD. Patients who develop symptoms of unexplained hyperammonemic encephalopathy while receiving valproate therapy should receive prompt treatment (including discontinuation of valproate therapy) and be evaluated for underlying urea cycle disorders [see Contraindications (4) and Warnings and Precautions (5.7)].
5.5Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including divalproex sodium extended-release tablets, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
Table 2 shows absolute and relative risk by indication for all evaluated AEDs.
| Indication | Placebo Patients with Events
Per 1000 Patients | Drug Patients with Events
Per 1000 Patients | Relative Risk: Incidence of Events
in Drug Patients/Incidence in Placebo Patients | Risk Difference: Additional Drug Patients with Events
Per 1000 Patients |
|---|---|---|---|---|
| Epilepsy | 1.0 | 3.4 | 3.5 | 2.4 |
| Psychiatric | 5.7 | 8.5 | 1.5 | 2.9 |
| Other | 1.0 | 1.8 | 1.9 | 0.9 |
| Total | 2.4 | 4.3 | 1.8 | 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing divalproex sodium extended-release tablets or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
5.6Thrombocytopenia
The frequency of adverse effects (particularly elevated liver enzymes and thrombocytopenia) may be dose-related. In a clinical trial of valproate as monotherapy in patients with epilepsy, 34/126 patients (27%) receiving approximately 50 mg/kg/day on average, had at least one value of platelets ≤ 75 x 10
9/L. Approximately half of these patients had treatment discontinued, with return of platelet counts to normal. In the remaining patients, platelet counts normalized with continued treatment. In this study, the probability of thrombocytopenia appeared to increase significantly at total valproate concentrations of ≥ 110 mcg/mL (females) or ≥ 135 mcg/mL (males). The therapeutic benefit which may accompany the higher doses should therefore be weighed against the possibility of a greater incidence of adverse effects.
Because of reports of thrombocytopenia, inhibition of the secondary phase of platelet aggregation, and abnormal coagulation parameters, (e.g., low fibrinogen), platelet counts and coagulation tests are recommended before initiating therapy and at periodic intervals. It is recommended that patients receiving divalproex sodium extended release tablets be monitored for platelet count and coagulation parameters prior to planned surgery. Evidence of hemorrhage, bruising, or a disorder of hemostasis/coagulation is an indication for reduction of the dosage or withdrawal of therapy.
5.7Hyperammonemia
Hyperammonemia has been reported in association with valproate therapy and may be present despite normal liver function tests. In patients who develop unexplained lethargy and vomiting or changes in mental status, hyperammonemic encephalopathy should be considered and an ammonia level should be measured. Hyperammonemia should also be considered in patients who present with hypothermia [see Warnings and Precautions (5.9)]. If ammonia is increased, valproate therapy should be discontinued. Appropriate interventions for treatment of hyperammonemia should be initiated, and such patients should undergo investigation for underlying urea cycle disorders [see Contraindications and Warnings and Precautions (4, 5.4, 5.8)].
During the placebo controlled pediatric mania trial, one (1) in twenty (20) adolescents (5%) treated with valproate developed increased plasma ammonia levels compared to no (0) patients treated with placebo.
Asymptomatic elevations of ammonia are more common and when present, require close monitoring of plasma ammonia levels. If the elevation persists, discontinuation of valproate therapy should be considered.
5.8Hyperammonemia and Encephalopathy associated with Concomitant Topiramate Use
Concomitant administration of topiramate and valproic acid has been associated with hyperammonemia with or without encephalopathy in patients who have tolerated either drug alone. Clinical symptoms of hyperammonemic encephalopathy often include acute alterations in level of consciousness and/or cognitive function with lethargy or vomiting. Hypothermia can also be a manifestation of hyperammonemia [see Warnings and Precautions (5.9)]. In most cases, symptoms and signs abated with discontinuation of either drug. This adverse event is not due to a pharmacokinetic interaction. It is not known if topiramate monotherapy is associated with hyperammonemia. Patients with inborn errors of metabolism or reduced hepatic mitochondrial activity may be at an increased risk for hyperammonemia with or without encephalopathy. Although not studied, an interaction of topiramate and valproic acid may exacerbate existing defects or unmask deficiencies in susceptible persons. In patients who develop unexplained lethargy, vomiting, or changes in mental status, hyperammonemic encephalopathy should be considered and an ammonia level should be measured. [see Contraindications (4) and Warnings and Precautions (5.7)].
5.9Hypothermia
Hypothermia,defined as an unintentional drop in body core temperature to <35 0C (95 0F), has been reported in association with valproate therapy both in conjunction with and in the absence of hyperammonemia. This adverse reaction can also occur in patients using concomitant topiramate with valproate after starting topiramate treatment or after increasing the daily dose of topiramate [see Drug Interactions (7.3)]. Consideration should be given to stopping valproate in patients who develop hypothermia, which may be manifested by a variety of clinical abnormalities including lethargy, confusion, coma, and significant alterations in other major organ systems such as the cardiovascular and respiratory systems. Clinical management and assessment should include examination of blood ammonia levels.
5.10Multi-Organ Hypersensitivity Reactions
Multi-organ hypersensitivity reactions have been rarely reported in close temporal association to the initiation of valproate therapy in adult and pediatric patients (median time to detection 21 days: range 1 to 40 days). Although there have been a limited number of reports, many of these cases resulted in hospitalization and at least one death has been reported. Signs and symptoms of this disorder were diverse; however, patients typically, although not exclusively, presented with fever and rash associated with other organ system involvement. Other associated manifestations may include lymphadenopathy, hepatitis, liver function test abnormalities, hematological abnormalities (e.g., eosinophilia, thrombocytopenia, neutropenia), pruritis, nephritis, oliguria, hepato-renal syndrome, arthralgia, and asthenia. Because the disorder is variable in its expression, other organ system symptoms and signs, not noted here, may occur. If this reaction is suspected, valproate should be discontinued and an alternative treatment started. Although the existence of cross sensitivity with other drugs that produce this syndrome is unclear, the experience amongst drugs associated with multi-organ hypersensitivity would indicate this to be a possibility.
5.11Interaction with Carbapenem Antibiotics
Carbapenem antibiotics (ertapenem, imipenem, meropenem) may reduce serum valproic acid concentrations to subtherapeutic levels, resulting in loss of seizure control. Serum valproic acid concentrations should be monitored frequently after initiating carbapenem therapy. Alternative antibacterial or anticonvulsant therapy should be considered if serum valproic acid concentrations drop significantly or seizure control deteriorates [see Drug Interactions (7.1)].
5.12Somnolence in the Elderly
In a double-blind, multicenter trial of valproate in elderly patients with dementia (mean age = 83 years), doses were increased by 125 mg per day to a target dose of 20 mg per kg per day. A significantly higher proportion of valproate patients had somnolence compared to placebo, and although not statistically significant, there was a higher proportion of patients with dehydration. Discontinuations for somnolence were also significantly higher than with placebo. In some patients with somnolence (approximately one-half), there was associated reduced nutritional intake and weight loss. There was a trend for the patients who experienced these events to have a lower baseline albumin concentration, lower valproate clearance, and a higher BUN. In elderly patients, dosage should be increased more slowly and with regular monitoring for fluid and nutritional intake, dehydration, somnolence, and other adverse reactions. Dose reductions or discontinuation of valproate should be considered in patients with decreased food or fluid intake and in patients with excessive somnolence [see Dosage and Administration (2.4)].
5.13Monitoring: Drug Plasma Concentration
Since valproic acid may interact with concurrently administered drugs which are capable of enzyme induction, periodic plasma concentration determinations of valproate and concomitant drugs are recommended during the early course of therapy. [see Drug Interactions (7)].
5.14Effect on Ketone and Thyroid function Tests
Valproate is partially eliminated in the urine as a keto-metabolite which may lead to a false interpretation of the urine ketone test.
There have been reports of altered thyroid function tests associated with valproate. The clinical significance of these is unknown.
5.15Effect on HIV and CMV Viruses Replication
There are in vitro studies that suggest valproate stimulates the replication of the HIV and CMV viruses under certain experimental conditions.The clinical consequence,if any, is not known. Additionally,the relevance of these in vitro findings is uncertain for patients receiving maximally suppressive antiretroviral therapy. Nevertheless, these data should be borne in mind when interpreting the results from regular monitoring of the viral load in HIVinfected patients receivingvalproate or when following CMV infected patients clinically.
6ADVERSE REACTIONS
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Information on pediatric adverse reactions is presented in section 8.
6.1Mania
The incidence of treatment-emergent events has been ascertained based on combined data from two placebo-controlled clinical trials of divalproex sodium extended-release tablets in the treatment of manic episodes associated with bipolar disorder.
Table 3 summarizes those adverse reactions reported for patients in these trials where the incidence rate in the divalproex sodium extended-release tablets-treated group was greater than 5% and greater than the placebo incidence.
| Adverse Event | Divalproex Sodium Extended-Release Tablets (n=338) | Placebo (n=263) |
|---|---|---|
| Somnolence | 26% | 14% |
| Dyspepsia | 23% | 11% |
| Nausea | 19% | 13% |
| Vomiting | 13% | 5% |
| Diarrhea | 12% | 8% |
| Dizziness | 12% | 7% |
| Pain | 11% | 10% |
| Abdominal Pain | 10% | 5% |
| Accidental injury | 6% | 5% |
| Asthenia | 6% | 5% |
| Pharyngitis | 6% | 5% |
1The following adverse reactions/event occurred at an equal or greater incidence for placebo than for divalproex sodium extended-release tablets: headache
The following additional adverse reactions were reported by greater than 1% but not more than 5% of the divalproex sodium extended-release tablets-treated patients in controlled clinical trials:
Body as a Whole: Back Pain, Flu Syndrome, Infection, Infection Fungal
Cardiovascular System: Hypertension
Digestive System: Constipation, Dry Mouth, Flatulence
Hemic and Lymphatic System: Ecchymosis
Metabolic and Nutritional Disorders: Peripheral Edema
Musculoskeletal System: Myalgia
Nervous System: Abnormal Gait, Hypertonia, Tremor
Respiratory System: Rhinitis
Skin and Appendages: Pruritus, Rash
Special Senses: Conjunctivitis
Urogenital System: Urinary Tract Infection, Vaginitis
6.2 Epilepsy
Based on a placebo-controlled trial of adjunctive therapy for treatment of complex partial seizures, divalproex sodium delayed-release tablets were generally well tolerated with most adverse reactions rated as mild to moderate in severity. Intolerance was the primary reason for discontinuation in the divalproex sodium delayed-release tablet-treated patients (6%), compared to 1% of placebo-treated patients.
Table 4 lists treatment-emergent adverse reactions which were reported by ≥ 5% of divalproex sodium delayed-release tablet-treated patients and for which the incidence was greater than in the placebo group, in the placebo-controlled trial of adjunctive therapy for treatment of complex partial seizures. Since patients were also treated with other antiepilepsy drugs, it is not possible, in most cases, to determine whether the following adverse reactions can be ascribed to divalproex sodium delayed-release tablets alone, or the combination of divalproex sodium delayed-release tablets and other antiepilepsy drugs.
| Body System/Event | Divalproex Sodium Delayed-Release
Tablets (%) (n=77) | Placebo (%)
(n=70) |
|---|---|---|
| Body as a Whole | ||
| Headache | 31 | 21 |
| Asthenia | 27 | 7 |
| Fever | 6 | 4 |
| Gastrointestinal System | ||
| Nausea | 48 | 14 |
| Vomiting | 27 | 7 |
| Abdominal pain | 23 | 6 |
| Diarrhea | 13 | 6 |
| Anorexia | 12 | 0 |
| Dyspepsia | 8 | 4 |
| Constipation | 5 | 1 |
| Nervous System | ||
| Somnolence | 27 | 11 |
| Tremor | 25 | 6 |
| Dizziness | 25 | 13 |
| Diplopia | 16 | 9 |
| Amblyopia/Blurred Vision | 12 | 9 |
| Ataxia | 8 | 1 |
| Nystagmus | 8 | 1 |
| Emotional Lability | 6 | 4 |
| Thinking Abnormal | 6 | 0 |
| Amnesia | 5 | 1 |
| Respiratory System | ||
| Flu Syndrome | 12 | 9 |
| Infection | 12 | 6 |
| Bronchitis | 5 | 1 |
| Rhinitis | 5 | 4 |
| Other | ||
| Alopecia | 6 | 1 |
| Weight Loss | 6 | 0 |
Table 5 lists treatment-emergent adverse reactions which were reported by ≥ 5% of patients in the high dose valproate group, and for which the incidence was greater than in the low dose group, in a controlled trial of divalproex sodium delayed-release tablets monotherapy treatment of complex partial seizures. Since patients were being titrated off another antiepilepsy drug during the first portion of the trial, it is not possible, in many cases, to determine whether the following adverse reactions can be ascribed to divalproex sodium delayed-release tablets alone, or the combination of valproate and other antiepilepsy drugs.
| Body System/Events | High Dose (%)
(n=131) | Low Dose (%)
(n=134) |
| Body as a Whole | ||
| Asthenia | 21 | 10 |
| Digestive System | ||
| Nausea | 34 | 26 |
| Diarrhea | 23 | 19 |
| Vomiting | 23 | 15 |
| Abdominal pain | 12 | 9 |
| Anorexia | 11 | 4 |
| Dyspepsia | 11 | 10 |
| Hemic/Lymphatic System | ||
| Thrombocytopenia | 24 | 1 |
| Ecchymosis | 5 | 4 |
| Metabolic/Nutritional | ||
| Weight Gain | 9 | 4 |
| Peripheral Edema | 8 | 3 |
| Nervous System | ||
| Tremor | 57 | 19 |
| Somnolence | 30 | 18 |
| Dizziness | 18 | 13 |
| Insomnia | 15 | 9 |
| Nervousnes | 11 | 7 |
| Amnesia | 7 | 4 |
| Nystagmus | 7 | 1 |
| Depression | 5 | 4 |
| Respiratory System | ||
| Infection | 20 | 13 |
| Pharynigits | 8 | 2 |
| Dyspnea | 5 | 1 |
| Skin and Appendages | ||
| Alopecia | 24 | 13 |
| Special Senses | ||
| Amblyopia/Blurred Vision | 8 | 4 |
| Tinnitus | 7 | 1 |
1Headache was the only adverse event that occurred in≥ 5% of patients in the high dose group and at an equal or greater incidence in the low dose group.
The following additional adverse reactions were reported by greater than 1% but less than 5% of the 358 patients treated with valproate in the controlled trials of complex partial seizures:
Body as a Whole: Back pain, chest pain, malaise.
Cardiovascular System: Tachycardia, hypertension, palpitation.
Digestive System: Increased appetite, flatulence, hematemesis, eructation, pancreatitis, periodontal abscess.
Hemic and Lymphatic System: Petechia.
Metabolic and Nutritional Disorders: SGOT increased, SGPT increased.
Musculoskeletal System: Myalgia, twitching, arthralgia, leg cramps, myasthenia.
Nervous System: Anxiety, confusion, abnormal gait, paresthesia, hypertonia, incoordination, abnormal dreams, personality disorder.
Respiratory System: Sinusitis, cough increased, pneumonia, epistaxis.
Skin and Appendages: Rash, pruritus, dry skin.
Special Senses: Taste perversion, abnormal vision, deafness, otitis media.
Urogenital System: Urinary incontinence, vaginitis, dysmenorrhea, amenorrhea, urinary frequency.
6.3Migraine
Based on two placebo-controlled clinical trials and their long term extension, valproate was generally well tolerated with most adverse reactions rated as mild to moderate in severity. Of the 202 patients exposed to valproate in the placebo-controlled trials, 17% discontinued for intolerance. This is compared to a rate of 5% for the 81 placebo patients. Including the long term extension study, the adverse reactions reported as the primary reason for discontinuation by ≥ 1% of 248 valproate-treated patients were alopecia (6%), nausea and/or vomiting (5%), weight gain (2%), tremor (2%), somnolence (1%), elevated SGOT and/or SGPT (1%), and depression (1%).
Table 6 includes those adverse reactions reported for patients in the placebo-controlled trial where the incidence rate in the divalproex sodium extended-release tablet-treated group was greater than 5% and was greater than that for placebo patients.
| Body System Event | Divalproex Sodium Extended- Release
(n=122) | Placebo
(n=115) |
|---|---|---|
| Gastrointestinal System | ||
| Nausea | 15% | 9% |
| Dyspepsia | 7% | 4% |
| Diarrhea | 7% | 3% |
| Vomiting | 7% | 2% |
| Abdominal pain | 7% | 5% |
| Nervous System | ||
| Somnolence | 7% | 2% |
| Other | ||
| Infection | 15% | 14% |
1The following adverse reactions occurred in greater than 5% of divalproex sodium extended-release tablet-treated patients and at a greater incidence for placebo than for divalproex sodium extended-release tablets: asthenia and flu syndrome.
The following additional adverse reactions were reported by greater than 1% but not more than 5% of divalproex sodium extended-release tablet-treated patients and with a greater incidence than placebo in the placebo-controlled clinical trial for migraine prophylaxis:
Body as a Whole :Accidental injury, viral infection.
Digestive System :Increased appetite, tooth disorder.
Metabolic and Nutritional Disorders :Edema, weight gain.
Nervous System :Abnormal gait, dizziness, hypertonia, insomnia, nervousness, tremor, vertigo.
Respiratory System :Pharyngitis, rhinitis.
Skin and Appendages :Rash.
Special Senses :Tinnitus.
Table 7 includes those adverse reactions reported for patients in the placebo-controlled trials where the incidence rate in the valproate-treated group was greater than 5% and was greater than that for placebo patients.
| Body System
Reaction | Divalproex Sodium Delayed-Release Tablets
(n=202) | Placebo
(n=81) |
|---|---|---|
| Gastrointestinal System | ||
| Nausea | 31% | 10% |
| Dyspepsia | 13% | 9% |
| Diarrhea | 12% | 7% |
| Vomiting | 11% | 1% |
| Abdominal pain | 9% | 4% |
| Increased appetite | 6% | 4% |
| Nervous System | ||
| Asthenia | 20% | 9% |
| Somnolence | 17% | 5% |
| Dizziness | 12% | 6% |
| Tremor | 9% | 0% |
| Other | ||
| Weight gain | 8% | 2% |
| Back pain | 8% | 6% |
| Alopecia | 7% | 1% |
1The following adverse reactions occurred in greater than 5% of divalproex sodium delayed-release tablet-treated patients and at a greater incidence for placebo than for divalproex sodium delayed-release tablets: flu syndrome and pharyngitis.
The following additional adverse reactions were reported by greater than 1% but not more than 5% of the 202 valproate-treated patients in the controlled clinical trials:
Body as a Whole: Chest pain.
Cardiovascular System: Vasodilatation.
Digestive System: Constipation, dry mouth, flatulence, and stomatitis.
Hemic and Lymphatic System: Ecchymosis.
Metabolic and Nutritional Disorders: Peripheral edema.
Musculoskeletal System: Leg cramps.
Nervous System: Abnormal dreams, confusion, paresthesia, speech disorder, and thinking abnormalities.
Respiratory System: Dyspnea, and sinusitis.
Skin and Appendages: Pruritus.
Urogenital System: Metrorrhagia.
6.4Other Patient Populations
Mania
The following adverse reactions not listed previously were reported by greater than 1% of divalproex sodium delayed-release tablets-treated patients and with a greater incidence than placebo in placebo-controlled trials of manic episodes associated with bipolar disorder:
Body as a Whole: Chills, chills and fever, drug level increased, neck rigidity.
Cardiovascular System: Arrhythmia, hypotension, postural hypotension.
Digestive System: Dysphagia, fecal incontinence, gastroenteritis, glossitis, gum hemorrhage, mouth ulceration.
Hemic and Lymphatic System: Anemia, bleeding time increased, leucopenia.
Metabolic and Nutritional Disorders: Hypoproteinemia.
Musculoskeletal System: Arthrosis.
Nervous System: Agitation, catatonic reaction, dysarthria, hallucinations, hypokinesia, psychosis, reflexes increased, sleep disorder, tardive dyskinesia.
Respiratory System: Hiccup.
Skin and Appendages: Discoid lupus erythematosus, erythema nodosum, furunculosis, maculopapular rash, seborrhea, sweating, vesiculobullous rash.
Special Senses: Conjunctivitis, dry eyes, eye disorder, eye pain, photophobia, taste perversion.
Urogenital System: Cystitis, menstrual disorder.
Epilepsy
Adverse reactions that have been reported with all dosage forms of valproate from epilepsy trials, spontaneous reports, and other sources are listed below by body system.
Gastrointestinal
The most commonly reported side effects at the initiation of therapy are nausea, vomiting, and indigestion. These effects are usually transient and rarely require discontinuation of therapy. Diarrhea, abdominal cramps, and constipation have been reported. Both anorexia with some weight loss and increased appetite with weight gain have also been reported. In some patients, many of whom have functional or anatomic (including ileostomy or colostomy) gastrointestinal disorders with shortened GI transit times, there have been postmarketing reports of divalproex sodium extended release tablets in stool.
CNS Effects
Sedative effects have occurred in patients receiving valproate alone but occur most often in patients receiving combination therapy. Sedation usually abates upon reduction of other antiepileptic medication. Tremor (may be dose-related), hallucinations, ataxia, headache, nystagmus, diplopia, asterixis, "spots before eyes", dysarthria, dizziness, confusion, hypesthesia, vertigo, incoordination, and parkinsonism have been reported with the use of valproate. Rare cases of coma have occurred in patients receiving valproate alone or in conjunction with phenobarbital. In rare instances encephalopathy with or without fever has developed shortly after the introduction of valproate monotherapy without evidence of hepatic dysfunction or inappropriately high plasma valproate levels. Although recovery has been described following drug withdrawal, there have been fatalities in patients with hyperammonemic encephalopathy, particularly in patients with underlying urea cycle disorders [see Warnings and Precautions (5.4)].
Several reports have noted reversible cerebral atrophy and dementia in association with valproate therapy.
Dermatologic
Transient hair loss, skin rash, photosensitivity, generalized pruritus, erythema multiforme, and Stevens-Johnson syndrome. Rare cases of toxic epidermal necrolysis have been reported including a fatal case in a 6 month old infant taking valproate and several other concomitant medications. An additional case of toxic epidermal necrosis resulting in death was reported in a 35 year old patient with AIDS taking several concomitant medications and with a history of multiple cutaneous drug reactions. Serious skin reactions have been reported with concomitant administration of lamotrigine and valproate [see Drug Interactions (7)].
Psychiatric
Emotional upset, depression, psychosis, aggression, hyperactivity, hostility, and behavioral deterioration.
Musculoskeletal
Weakness.
Hematologic
Thrombocytopenia and inhibition of the secondary phase of platelet aggregation may be reflected in altered bleeding time, petechiae, bruising, hematoma formation, epistaxis, and frank hemorrhage [see Warnings and Precautions (5.6) and Drug Interactions (7)]. Relative lymphocytosis, macrocytosis, hypofibrinogenemia, leukopenia, eosinophilia, anemia including macrocytic with or without folate deficiency, bone marrow suppression, pancytopenia, aplastic anemia, agranulocytosis, and acute intermittent porphyria.
Hepatic
Minor elevations of transaminases (eg, SGOT and SGPT) and LDH are frequent and appear to be dose-related. Occasionally, laboratory test results include increases in serum bilirubin and abnormal changes in other liver function tests. These results may reflect potentially serious hepatotoxicity [see Warnings and Precautions (5.1].
Endocrine
Irregular menses, secondary amenorrhea, breast enlargement, galactorrhea, and parotid gland swelling. Abnormal thyroid function tests [see Warnings and Precautions (5.13)].
There have been rare spontaneous reports of polycystic ovary disease. A cause and effect relationship has not been established.
Pancreatic
Acute pancreatitis including fatalities [see Warnings and Precautions (5.3)].
Metabolic
Hyperammonemia [see Warnings and Precautions (5.7), hyponatremia, and inappropriate ADH secretion.
There have been rare reports of Fanconi's syndrome occurring chiefly in children.
Decreased carnitine concentrations have been reported although the clinical relevance is undetermined.
Hyperglycinemia has occurred and was associated with a fatal outcome in a patient with preexistent nonketotic hyperglycinemia.
Genitourinary
Enuresis and urinary tract infection.
Special Senses
Hearing loss, either reversible or irreversible, has been reported; however, a cause and effect relationship has not been established. Ear pain has also been reported.
Other
Allergic reaction, anaphylaxis, edema of the extremities, lupus erythematosus, bone pain, cough increased, pneumonia, otitis media, bradycardia, cutaneous vasculitis, fever, and hypothermia.
7DRUG INTERACTIONS
7.1Effects of Co-Administered Drugs on Valproate Clearance Valproate Clearance
Drugs that affect the level of expression of hepatic enzymes, particularly those that elevate levels of glucuronosyltransferases, may increase the clearance of valproate. For example, phenytoin, carbamazepine, and phenobarbital (or primidone) can double the clearance of valproate. Thus, patients on monotherapy will generally have longer half-lives and higher concentrations than patients receiving polytherapy with antiepilepsy drugs.
In contrast, drugs that are inhibitors of cytochrome P450 isozymes, e.g., antidepressants, may be expected to have little effect on valproate clearance because cytochrome P450 microsomal mediated oxidation is a relatively minor secondary metabolic pathway compared to glucuronidation and beta-oxidation.
Because of these changes in valproate clearance, monitoring of valproate and concomitant drug concentrations should be increased whenever enzyme inducing drugs are introduced or withdrawn.
The following list provides information about the potential for an influence of several commonly prescribed medications on valproate pharmacokinetics. The list is not exhaustive nor could it be, since new interactions are continuously being reported.
Drugs for which a potentially important interaction has been observed
Aspirin
A study involving the co-administration of aspirin at antipyretic doses (11 to 16 mg/kg) with valproate to pediatric patients (n=6) revealed a decrease in protein binding and an inhibition of metabolism of valproate. Valproate free fraction was increased 4-fold in the presence of aspirin compared to valproate alone. The β-oxidation pathway consisting of 2-E-valproic acid, 3-OH-valproic acid, and 3-keto valproic acid was decreased from 25% of total metabolites excreted on valproate alone to 8.3% in the presence of aspirin. Whether or not the interaction observed in this study applies to adults is unknown, but caution should be observed if valproate and aspirin are to be co-administered.
Carbapenem antibiotics
A clinically significant reduction in serum valproic acid concentration has been reported in patients receiving carbapenem antibiotics (ertapenem, imipenem, meropenem) and may result in loss of seizure control. The mechanism of this interaction is not well understood. Serum valproic acid concentrations should be monitored frequently after initiating carbapenem therapy. Alternative antibacterial or anticonvulsant therapy should be considered if serum valproic acid concentrations drop significantly or seizure control deteriorates [see Warnings and Precautions (5.11)].
Felbamate
A study involving the co-administration of 1200 mg/day of felbamate with valproate to patients with epilepsy (n=10) revealed an increase in mean valproate peak concentration by 35% (from 86 to 115 mcg/mL) compared to valproate alone. Increasing the felbamate dose to 2400 mg/day increased the mean valproate peak concentration to 133 mcg/mL (another 16% increase). A decrease in valproate dosage may be necessary when felbamate therapy is initiated.
Rifampin
A study involving the administration of a single dose of valproate (7 mg/kg) 36 hours after 5 nights of daily dosing with rifampin (600 mg) revealed a 40% increase in the oral clearance of valproate. Valproate dosage adjustment may be necessary when it is coadministered with rifampin.
Drugs for which either no interaction or a likely clinically unimportant interaction has been observed
Antacids
A study involving the co-administration of valproate 500 mg with commonly administered antacids (Maalox, Trisogel, and Titralac - 160 mEq doses) did not reveal any effect on the extent of absorption of valproate.
Chlorpromazine
A study involving the administration of 100 to 300 mg/day of chlorpromazine to schizophrenic patients already receiving valproate (200 mg BID) revealed a 15% increase in trough plasma levels of valproate.
Haloperidol
A study involving the administration of 6 to 10 mg/day of haloperidol to schizophrenic patients already receiving valproate (200 mg BID) revealed no significant changes in valproate trough plasma levels.
Cimetidine and Ranitidine
Cimetidine and ranitidine do not affect the clearance of valproate.
7.2Effects of Valproate on Other Drugs
Valproate has been found to be a weak inhibitor of some P450 isozymes, epoxide hydrase, and glucuronosyltransferases.
The following list provides information about the potential for an influence of valproate co-administration on the pharmacokinetics or pharmacodynamics of several commonly prescribed medications. The list is not exhaustive, since new interactions are continuously being reported.
Drugs for which a potentially important valproate interaction has been observed
Amitriptyline/Nortriptyline
Administration of a single oral 50 mg dose of amitriptyline to 15 normal volunteers (10 males and 5 females) who received valproate (500 mg BID) resulted in a 21% decrease in plasma clearance of amitriptyline and a 34% decrease in the net clearance of nortriptyline. Rare postmarketing reports of concurrent use of valproate and amitriptyline resulting in an increased amitriptyline level have been received. Concurrent use of valproate and amitriptyline has rarely been associated with toxicity. Monitoring of amitriptyline levels should be considered for patients taking valproate concomitantly with amitriptyline. Consideration should be given to lowering the dose of amitriptyline/nortriptyline in the presence of valproate.
Carbamazepine/carbamazepine-10,11-Epoxide
Serum levels of carbamazepine (CBZ) decreased 17% while that of carbamazepine-10,11-epoxide (CBZ-E) increased by 45% upon co-administration of valproate and CBZ to epileptic patients.
Clonazepam
The concomitant use of valproic acid and clonazepam may induce absence status in patients with a history of absence type seizures.
Diazepam
Valproate displaces diazepam from its plasma albumin binding sites and inhibits its metabolism. Co-administration of valproate (1500 mg daily) increased the free fraction of diazepam (10 mg) by 90% in healthy volunteers (n=6). Plasma clearance and volume of distribution for free diazepam were reduced by 25% and 20%, respectively, in the presence of valproate. The elimination half-life of diazepam remained unchanged upon addition of valproate.
Ethosuximide
Valproate inhibits the metabolism of ethosuximide. Administration of a single ethosuximide dose of 500 mg with valproate (800 to 1600 mg/day) to healthy volunteers (n=6) was accompanied by a 25% increase in elimination half-life of ethosuximide and a 15% decrease in its total clearance as compared to ethosuximide alone. Patients receiving valproate and ethosuximide, especially along with other anticonvulsants, should be monitored for alterations in serum concentrations of both drugs.
Lamotrigine
In a steady-state study involving 10 healthy volunteers, the elimination half-life of lamotrigine increased from 26 to 70 hours with valproate co-administration (a 165% increase). The dose of lamotrigine should be reduced when co-administered with valproate. Serious skin reactions (such as Stevens-Johnson Syndrome and toxic epidermal necrolysis) have been reported with concomitant lamotrigine and valproate administration. See lamotrigine package insert for details on lamotrigine dosing with concomitant valproate administration.
Phenobarbital
Valproate was found to inhibit the metabolism of phenobarbital. Co-administration of valproate (250 mg BID for 14 days) with phenobarbital to normal subjects (n=6) resulted in a 50% increase in half-life and a 30% decrease in plasma clearance of phenobarbital (60 mg single-dose). The fraction of phenobarbital dose excreted unchanged increased by 50% in presence of valproate.
There is evidence for severe CNS depression, with or without significant elevations of barbiturate or valproate serum concentrations. All patients receiving concomitant barbiturate therapy should be closely monitored for neurological toxicity. Serum barbiturate concentrations should be obtained, if possible, and the barbiturate dosage decreased, if appropriate.
Primidone, which is metabolized to a barbiturate, may be involved in a similar interaction with valproate.
Phenytoin
Valproate displaces phenytoin from its plasma albumin binding sites and inhibits its hepatic metabolism. Co-administration of valproate (400 mg TID) with phenytoin (250 mg) in normal volunteers (n=7) was associated with a 60% increase in the free fraction of phenytoin. Total plasma clearance and apparent volume of distribution of phenytoin increased 30% in the presence of valproate. Both the clearance and apparent volume of distribution of free phenytoin were reduced by 25%.
In patients with epilepsy, there have been reports of breakthrough seizures occurring with the combination of valproate and phenytoin. The dosage of phenytoin should be adjusted as required by the clinical situation.
Tolbutamide
From in vitro experiments, the unbound fraction of tolbutamide was increased from 20% to 50% when added to plasma samples taken from patients treated with valproate. The clinical relevance of this displacement is unknown.
Warfarin
In an in vitro study, valproate increased the unbound fraction of warfarin by up to 32.6%. The therapeutic relevance of this is unknown; however, coagulation tests should be monitored if valproic acid therapy is instituted in patients taking anticoagulants.
Zidovudine
In six patients who were seropositive for HIV, the clearance of zidovudine (100 mg q8h) was decreased by 38% after administration of valproate (250 or 500 mg q8h); the half-life of zidovudine was unaffected.
Drugs for which either no interaction or a likely clinically unimportant interaction has been observed
Acetaminophen
Valproate had no effect on any of the pharmacokinetic parameters of acetaminophen when it was concurrently administered to three epileptic patients.
Clozapine
In psychotic patients (n=11), no interaction was observed when valproate was co-administered with clozapine.
Lithium
Co-administration of valproate (500 mg BID) and lithium carbonate (300 mg TID) to normal male volunteers (n=16) had no effect on the steady-state kinetics of lithium.
Lorazepam
Concomitant administration of valproate (500 mg BID) and lorazepam (1 mg BID) in normal male volunteers (n=9) was accompanied by a 17% decrease in the plasma clearance of lorazepam.
Oral Contraceptive Steroids
Administration of a single-dose of ethinyloestradiol (50 mcg)/levonorgestrel (250 mcg) to 6 women on valproate (200 mg BID) therapy for 2 months did not reveal any pharmacokinetic interaction.
7.3Topiramate
Concomitant administration of valproic acid and topiramate has been associated with hyperammonemia with and without encephalopathy [see Contraindications and Warnings and Precautions (4, 5.7, 5.8)].
Concomitant administration of topiramate with valproic acid has also been associated with hypothermia in patients who have tolerated either drug alone. It may be prudent to examine blood ammonia levels in patients in whom the onset of hypothermia has been reported [see Warnings and Precautions (5.7, 5.9)].
8USE IN SPECIFIC POPULATIONS
8.1Pregnancy
Teratogenic Effects: Pregnancy Category D.
Use of divalproex sodium extended-release tablets during pregnancy can cause congenital malformations including neural tube defects. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Divalproex sodium extended-release tablets should be considered for women of childbearing potential only after the risks have been thoroughly discussed with the patient and weighed against the potential benefits of treatment.
Human Data
Congenital Malformations
The North American Antiepileptic Drug Pregnancy Registry reported 16 cases of congenital malformations among the offspring of 149 women with epilepsy who were exposed to valproic acid monotherapy during the first trimester of pregnancy at doses of approximately 1,000 mg per day, for a prevalence rate of 10.7% (95% CI 6.3%-16.9%). Three of the 149 offspring (2%) had neural tube defects and 6 of the 149 (4%) had less severe malformations. Among epileptic women who were exposed to other antiepileptic drug monotherapies during pregnancy (1,048 patients) the malformation rate was 2.9% (95% CI 2.0% to 4.1%). There was a 4-fold increase in congenital malformations among infants with valproic acid-exposed mothers compared with those treated with other antiepileptic monotherapies as a group (Odds Ratio 4.0; 95% CI 2.1 to 7.4). This increased risk does not reflect a comparison versus any specific antiepileptic drug, but the risk versus the heterogeneous group of all other antiepileptic drug monotherapies combined. The increased teratogenic risk from valproic acid in women with epilepsy is expected to be reflected in an increased risk in other indications (e.g., migraine or bipolar disorder).
The strongest association of maternal valproate usage with congenital malformations is with neural tube defects (as discussed under the next subheading). However, other congenital anomalies (e.g. craniofacial defects, cardiovascular malformations and anomalies involving various body systems), compatible and incompatible with life, have been reported. Sufficient data to determine the incidence of these congenital anomalies are not available.
Neural Tube Defects
The incidence of neural tube defects in the fetus is increased in mothers receiving valproate during the first trimester of pregnancy. The Centers for Disease Control (CDC) has estimated the risk of valproic acid exposed women having children with spina bifida to be approximately 1 to 2%. The American College of Obstetricians and Gynecologists (ACOG) estimates the general population risk for congenital neural tube defects as 0.14% to 0.2%.
Tests to detect neural tube and other defects using currently accepted procedures should be considered a part of routine prenatal care in pregnant women receiving valproate.
Evidence suggests that pregnant women who receive folic acid supplementation may be at decreased risk for congenital neural tube defects in their offspring compared to pregnant women not receiving folic acid. Whether the risk of neural tube defects in the offspring of women receiving valproate specifically is reduced by folic acid supplementation is unknown. Dietary folic acid supplementation both prior to and during pregnancy should be routinely recommended to patients contemplating pregnancy.
Other Adverse Pregnancy Effects
Patients taking valproate may develop clotting abnormalities [see Warnings and Precautions (5.6)]. A patient who had low fibrinogen when taking multiple anticonvulsants including valproate gave birth to an infant with afibrinogenemia who subsequently died of hemorrhage. If valproate is used in pregnancy, the clotting parameters should be monitored carefully.
Patients taking valproate may develop hepatic failure [see Warnings and Precautions (5.1)]. Fatal hepatic failures, in a newborn and in an infant, have been reported following the maternal use of valproate during pregnancy.
Animal Data
Reproduction studies have demonstrated valproate-induced teratogenicity. Increased incidences of malformations, as well as intrauterine growth retardation and death, have been observed in mice, rats, rabbits, and monkeys following prenatal exposure to valproate. Malformations of the skeletal system are the most common structural abnormalities produced in experimental animals; however, neural tube closure defects were observed in mice exposed during organogenesis to maternal plasma valproate concentrations 2.3 times the upper limit of the human therapeutic range.
In pregnant rats, oral administration during organogenesis of a dose≥0.5 times the maximum recommended daily human dose on a mg/m
2 basis (MRHD) produced malformations (e.g. skeletal, cardiac, and urogenital) and growth retardation in the offspring. These doses resulted in peak maternal plasma valproate levels of ≥3.4 times the upper limit of the human therapeutic range. Behavioral deficits have been reported in the offspring of rats given 0.5 times the MRHD on a mg/m
2 basis throughout most of pregnancy.
Valproate produced skeletal and visceral malformations in the offspring of pregnant rabbits given an oral dose approximately 2 times the MRHD on a mg/m
2 basis during organogenesis. Skeletal malformations, growth retardation, and death were observed in rhesus monkeys following an oral dose equal to the MRHD on a mg/m
2 basis during organogenesis. This dose resulted in peak maternal plasma valproate levels 2.8 times the upper limit of the human therapeutic range.
Registry
To provide information regarding the effects of in utero exposure to divalproex sodium extended-release tablets, healthcare providers are advised to recommend that pregnant patients taking divalproex sodium extended-release tablets enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
8.3Nursing Mothers
Valproate is excreted in breast milk. Concentrations in breast milk have been reported to be 1-10% of serum concentrations. Because of the potential for adverse reactions in a nursing infant, a decision between the physician and the patient should be made on whether to discontinue nursing or consider an alternative drug treatment for the mother, as appropriate
8.4Pediatric Use
Divalproex sodium was studied in seven pediatric clinical trials. Two of the pediatric studies were placebo-controlled to evaluate the efficacy of divalproex sodium extended-release tablets for the indications of mania (150 patients aged 10 to 17 years, 76 of whom were on divalproex sodium extended-release tablets) and migraine (304 patients aged 12 to 17 years, 231 of whom were on divalproex sodium extended-release tablets).
Mania
A single 4-week outpatient, double-blind, placebo controlled study of 150 patients aged 10-17 years of age with pediatric bipolar disorder was conducted to evaluate the efficacy of divalproex sodium extended-release tablets in the treatment of pediatric bipolar disorder. Initial daily doses of 15mg/kg (max. 750mg/day) and flexible dosing was used to achieve a clinical response and/or a target serum valproate level of 80-125 mcg/ml with a maximum allowable dose set at 35mg/kg. Patients on stimulant medications at screening were allowed to continue and maintain current stimulant doses during the trial provided that doses were clinically stable. The trial efficacy endpoint was change from baseline on the YMRS scale at final visit.
Results from the trial revealed that the mean maximum daily dose of 1457 mg (27.1 mg/kg) with a mean final serum valproate concentration of 80mcg/ml was attained in this clinical trial.
Efficacy was not established in this study.
Migraine Prophylaxis
A single, double-blind, placebo-controlled, parallel-group, four equal armed (placebo, 250 mg, 500 mg and 1,000 mg) trial was performed to evaluate the efficacy of divalproex sodium extended-release tablets in adolescent patients with migraine (304 patients, ages 12-17 years old). The study consisted of a 4 week baseline period followed by a 12 week experimental period (including an initial 2 week titration phase). The primary endpoint was the reduction from baseline in the 4 week migraine headache rate. Placebo was compared to each dose.
Efficacy was not established in this migraine study.
Epilepsy
Divalproex sodium extended-release tablet has not been proven to be safe and effective for epilepsy in children less than 10 years of age.
Pediatric Safety
Two six-month pediatric studies were conducted to evaluate the long-term safety of divalproex sodium extended-release tablets in the indication of mania (292 patients aged 10 to 17 years). Two twelve-month pediatric studies were conducted to evaluate the long-term safety of divalproex sodium extended-release tablets in the indication of migraine (353 patients aged 12 to 17 years). One twelve-month study was conducted to evaluate the safety of Divalproex Sodium Sprinkles Capsules in the indication of partial seizures (169 patients aged 3 to 10 years).
Safety Studies-Mania
Safety Study-Controlled Mania Trial
The incidence of treatment-emergent events for the pediatric population was based on the data from the single placebo controlled clinical trial of divalproex sodium extended-release tablets in the treatment of manic or mixed episodes associated with bipolar disorder.
Table 8 includes those adverse reactions reported for pediatric patients in the placebo-controlled mania trial where the incidence rate in the valproate-treated group was ≥5% and was at least twice the rate than that for placebo patients.
| Adverse reaction- prefered term | Divalproex sodium Extended-Release Tablets
(n=76) | Placebo
(n=74) |
|---|---|---|
| Nausea | 9% | 1% |
| Upper abdominal Pain | 8% | 1% |
| Somnolence | 7% | 1% |
| Increased Ammonia | 5% | 0% |
| Gastritis | 5% | 0% |
| Rash | 5% | 1% |
In addition, patients taking divalproex sodium extended-release tablets had a statistically significant 1.5 lbs mean increase in weight and 0.4 unit BMI mean increase from baseline values over placebo treated patients.
Safety Study-Open Label Mania Safety Data
In the two long-term (six month) safety studies in pediatric patients (n= 292) between the ages of 10 and 17 years old, no clinically meaningful differences in the adverse reaction profile were observed when compared to adults.
The safety and tolerability of divalproex sodium extended-release tablets in pediatric patients were shown to be comparable to those in adults [see Adverse Reactions (6.1, 6.2, 6.3).
Safety Study-Epilepsy (open label)
Safety and tolerability in this study was found comparable to that observed in adult epilepsy studies.
Safety Studies-Migraine (controlled and open label)
Safety and tolerability in this study was found comparable to that observed in adult migraine studies.
Prior Safety Experience
Experience has indicated that pediatric patients under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those with the aforementioned conditions [see Boxed Warning, Warnings and Precautions (5.1)]. When valproic acid is used in this patient group, it should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. Above the age of 2 years, experience in epilepsy has indicated that the incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
The variability in free fraction limits the clinical usefulness of monitoring total serum valproic acid concentrations. Interpretation of valproic acid concentrations in children should include consideration of factors that affect hepatic metabolism and protein binding.
The safety and effectiveness of valproic acid for the treatment of acute mania has not been established in individuals below the age of 18 years.
The safety and effectiveness of valproic acid for the prophylaxis of migraines has not been studied in individuals below the age of 12 years.
Nonclinical Developmental Toxicology
The basic toxicology and pathologic manifestations of valproate sodium in neonatal (4-day old) and juvenile (14-day old) rats are similar to those seen in young adult rats. However, additional findings, including renal alterations in juvenile rats and renal alterations and retinal dysplasia in neonatal rats, have been reported. These findings occurred at a dose approximately equal to the maximum recommended daily human dose (MRHD). They were not seen at a dose 0.4 times the MRHD.
8.5Geriatric Use
No patients above the age of 65 years were enrolled in double-blind prospective clinical trials of mania associated with bipolar illness. In a case review study of 583 patients, 72 patients (12%) were greater than 65 years of age. A higher percentage of patients above 65 years of age reported accidental injury, infection, pain, somnolence, and tremor.
Discontinuation of valproate was occasionally associated with the latter two events. It is not clear whether these events indicate additional risk or whether they result from preexisting medical illness and concomitant medication use among these patients.
A study of elderly patients with dementia revealed drug related somnolence and discontinuation for somnolence [see Warnings and Precautions (5.12)]. The starting dose should be reduced in these patients, and dosage reductions or discontinuation should be considered in patients with excessive somnolence [see Dosage and Administration (2.4)].
There is insufficient information available to discern the safety and effectiveness of valproic acid for the prophylaxis of migraines in patients over 65.
The capacity of elderly patients (age range: 68 to 89 years) to eliminate valproate has been shown to be reduced compared to younger adults (age range: 22 to 26 years) [see Clinical Pharmacology (12.3)].
10OVERDOSAGE
Over dosage with valproate may result in somnolence, heart block, and deep coma. Fatalities have been reported; however patients have recovered from valproate levels as high as 2120 mcg/mL.
In overdose situations, the fraction of drug not bound to protein is high and hemodialysis or tandem hemodialysis plus hemoperfusion may result in significant removal of drug. The benefit of gastric lavage or emesis will vary with the time since ingestion. General supportive measures should be applied with particular attention to the maintenance of adequate urinary output.
Naloxone has been reported to reverse the CNS depressant effects of valproate over dosage. Because naloxone could theoretically also reverse the antiepileptic effects of valproate, it should be used with caution in patients with epilepsy.
11DESCRIPTION
Divalproex sodium is a stable co-ordination compound comprised of sodium valproate and valproic acid in a 1:1 molar relationship and formed during the partial neutralization of valproic acid with 0.5 equivalent of sodium hydroxide.
Chemically it is designated as sodium hydrogen bis(2-propylpentanoate). Divalproex sodium has the following structure:
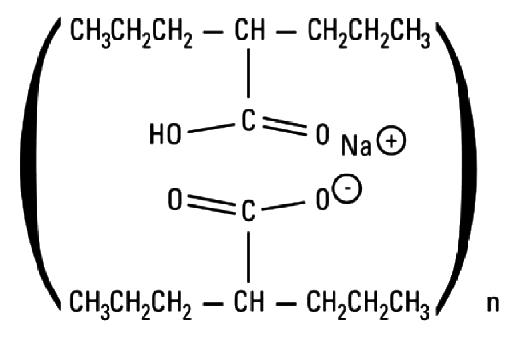
Divalproex sodium occurs as a white crystalline powder, with a characteristic odor.
Divalproex sodium extended-release tablets 250 and 500 mg are for oral administration. Divalproex sodium extended-release tablets contain divalproex sodium in a once-a-day extended-release formulation equivalent to 250 and 500 mg of valproic acid.
Inactive Ingredients
Divalproex sodium extended-release 250 and 500 mg tablets: microcrystalline cellulose, xanthan gum, hypromellose, glyceryl behenate, silicon dioxide, polyvinyl alcohol, talc, titanium dioxide, polyethylene glycol,lecithin.
In addition, 500 mg tablets contain iron oxide black.
12CLINICAL PHARMACOLOGY
12.1Mechanism of Action
Divalproex sodium dissociates to the valproate ion in the gastrointestinal tract. The mechanisms by which valproate exerts its therapeutic effects have not been established. It has been suggested that its activity in epilepsy is related to increased brain concentrations of gamma-aminobutyric acid (GABA).
12.2Pharmacodynamics
The relationship between plasma concentration and clinical response is not well documented. One contributing factor is the nonlinear, concentration dependent protein binding of valproate which affects the clearance of the drug. Thus, monitoring of total serum valproate may not provide a reliable index of the bioactive valproate species as protein binding may be affected by age and disease state (e.g. hepatic or renal insufficiency, hyperlipidemia).
Epilepsy
The therapeutic range in epilepsy is commonly considered to be 50 to 100 mcg/mL of total valproate, although some patients may be controlled with lower or higher plasma concentrations.
Mania
In placebo-controlled clinical trials of acute mania, patients were dosed to clinical response with trough plasma concentrations between 85 and 125 mcg/mL [see Dosage and Administration (2.1)].
12.3Pharmacokinetics
Absorption/Bioavailability
The absolute bioavailability of divalproex sodium extended release tablets administered as a single dose after a meal was approximately 90% relative to intravenous infusion.
When given in equal total daily doses, the bioavailability of divalproex sodium extended-release tablets is less than that of divalproex sodium delayed-release tablets. In five multiple-dose studies in healthy subjects (N=82) and in subjects with epilepsy (N=86), when administered under fasting and nonfasting conditions, divalproex sodium extended-release tablets given once daily produced an average bioavailability of 89% relative to an equal total daily dose of divalproex sodium delayed-release tablets given BID, TID, or QID. The median time to maximum plasma valproate concentrations (C
max) after divalproex sodium extended-release tablets administration ranged from 4 to 17 hours. After multiple once-daily dosing of divalproex sodium extended-release tablets, the peak-to-trough fluctuation in plasma valproate concentrations was 10-20% lower than that of regular divalproex sodium delayed-release tablets given BID, TID, or QID.
Conversion from Divalproex Sodium Delayed-Release Tablets to Divalproex Sodium Extended-Release Tablets
When divalproex sodium extended-release tablets are given in doses 8 to 20% higher than the total daily dose of divalproex sodium delayed-release tablets, the two formulations are bioequivalent. In two randomized, crossover studies, multiple daily doses of divalproex sodium delayed-release tablets were compared to 8 to 20% higher once daily doses of divalproex sodium extended-release tablets. In these two studies, divalproex sodium extended-release tablets and divalproex sodium delayed-release tablets regimens were equivalent with respect to area under the curve (AUC; a measure of the extent of bioavailability). Additionally, valproate C
max was lower, and C
min was either higher or not different, for divalproex sodium extended-release tablets relative to divalproex sodium delayed-release tablets regimens (see Table 9).
| Study
Population | Regimens | Relative | Bioavailability | |
| Divalproex Sodium Extended-Release Tablets
vs. Divalproex Sodium Delayed-Release Tablets | AUC 24 | C max | C min | |
| Healthy Volunteers (N=35) | 1000 and 1500 mgDivalproex sodium extended-release
tablets vs 875 and 1250 mg Divalproex sodium delayed-release tablets | 1.059 | 0.882 | 1.173 |
| Patients with epilepsy on concomitant
enzyme-inducing antiepilepsy drugs (N = 64) | 1000 and 5000 mgDivalproex sodium extended-release
tablets vs 875 and 4250 mg Divalproex sodium delayed-release tablets | 1.008 | 0.899 | 1.022 |
Concomitant antiepilepsy drugs (topiramate, phenobarbital, carbamazepine, phenytoin, and lamotrigine were evaluated) that induce the cytochrome P450 isozyme system did not significantly alter valproate bioavailability when converting between divalproex sodium delayed-release tablets and divalproex sodium extended-release tablets.
Distribution
Protein Binding
The plasma protein binding of valproate is concentration dependent and the free fraction increases from approximately 10% at 40 mcg/mL to 18.5% at 130 mcg/mL. Protein binding of valproate is reduced in the elderly, in patients with chronic hepatic diseases, in patients with renal impairment, and in the presence of other drugs (e.g., aspirin). Conversely, valproate may displace certain protein-bound drugs (e.g., phenytoin, carbamazepine, warfarin, and tolbutamide) [see Drug Interactions (7) for more detailed information on the pharmacokinetic interactions of valproate with other drugs].
CNS Distribution
Valproate concentrations in cerebrospinal fluid (CSF) approximate unbound concentrations in plasma (about 10% of total concentration).
Metabolism
Valproate is metabolized almost entirely by the liver. In adult patients on monotherapy, 30-50% of an administered dose appears in urine as a glucuronide conjugate. Mitochondrial beta -oxidation is the other major metabolic pathway, typically accounting for over 40% of the dose. Usually, less than 15-20% of the dose is eliminated by other oxidative mechanisms. Less than 3% of an administered dose is excreted unchanged in urine.
The relationship between dose and total valproate concentration is nonlinear; concentration does not increase proportionally with the dose, but rather, increases to a lesser extent due to saturable plasma protein binding. The kinetics of unbound drug are linear.
Elimination
Mean plasma clearance and volume of distribution for total valproate are 0.56 L/hr/1.73 m
2 and 11 L/1.73 m
2, respectively.
Mean plasma clearance and volume of distribution for free valproate are 4.6 L/hr/1.73 m
2 and 92 L/1.73 m
2. Mean terminal half-life for valproate monotherapy ranged from 9 to 16 hours following oral dosing regimens of 250 to 1000 mg.
The estimates cited apply primarily to patients who are not taking drugs that affect hepatic metabolizing enzyme systems. For example, patients taking enzyme-inducing antiepileptic drugs (carbamazepine, phenytoin, and phenobarbital) will clear valproate more rapidly. Because of these changes in valproate clearance, monitoring of antiepileptic concentrations should be intensified whenever concomitant antiepileptics are introduced or withdrawn.
Special Populations
Effect of Age
Pediatric
The valproate pharmacokinetic profile following administration of divalproex sodium extended-release tablets was characterized in a multiple-dose, nonfasting, open label, multi-center study in children and adolescents. Divalproex sodium extended-release tablets once daily doses ranged from 250-1750 mg. Once daily administration of divalproex sodium extended-release tablets in pediatric patients (10-17 years) produced plasma VPA concentration-time profiles similar to those that have been observed in adults.
Elderly
The capacity of elderly patients (age range: 68 to 89 years) to eliminate valproate has been shown to be reduced compared to younger adults (age range: 22 to 26 years). Intrinsic clearance is reduced by 39%; the free fraction is increased by 44%. Accordingly, the initial dosage should be reduced in the elderly [see Dosage and Administration (2.4)].
Effect of Sex
There are no differences in the body surface area adjusted unbound clearance between males and females (4.8±0.17 and 4.7±0.07 L/hr per 1.73 m
2, respectively).
Effect of Race
The effects of race on the kinetics of valproate have not been studied.
Effect of Disease
Liver Disease
Liver disease impairs the capacity to eliminate valproate. In one study, the clearance of free valproate was decreased by 50% in 7 patients with cirrhosis and by 16% in 4 patients with acute hepatitis, compared with 6 healthy subjects. In that study, the half-life of valproate was increased from 12 to 18 hours. Liver disease is also associated with decreased albumin concentrations and larger unbound fractions (2 to 2.6 fold increase) of valproate. Accordingly, monitoring of total concentrations may be misleading since free concentrations may be substantially elevated in patients with hepatic disease whereas total concentrations may appear to be normal [See Boxed Warning, Contraindications (4), Warnings and Precautions (5.1)].
Renal Disease
A slight reduction (27%) in the unbound clearance of valproate has been reported in patients with renal failure (creatinine clearance < 10 mL/minute); however, hemodialysis typically reduces valproate concentrations by about 20%. Therefore, no dosage adjustment appears to be necessary in patients with renal failure. Protein binding in these patients is substantially reduced; thus, monitoring total concentrations may be misleading.
13NONCLINICAL TOXICOLOGY
Enter section text here
13.1Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Valproic acid was administered orally to Sprague Dawley rats and ICR (HA/ICR) mice at doses of 80 and 170 mg/kg/day (approximately 10 to 50% of the maximum human daily dose on a mg/m2 basis) for two years. A variety of neoplasms were observed in both species. The primary findings were a statistically significant increase in the incidence of subcutaneous fibrosarcomas in high dose male rats receiving valproic acid and a statistically significant dose-related trend for benign pulmonary adenomas in male mice receiving valproic acid. The significance of these findings for humans is unknown.
Mutagenesis
Valproate was not mutagenic in an in vitro bacterial assay (Ames test), did not produce dominant lethal effects in mice, and did not increase chromosome aberration frequency in an in vivo cytogenetic study in rats. Increased frequencies of sister chromatid exchange (SCE) have been reported in a study of epileptic children taking valproate, but this association was not observed in another study conducted in adults. There is some evidence that increased SCE frequencies may be associated with epilepsy. The biological significance of an increase in SCE frequency is not known.
Fertility
Chronic toxicity studies in juvenile and adult rats and dogs demonstrated reduced spermatogenesis and testicular atrophy at oral doses of 400 mg/kg/day or greater in rats (approximately equivalent to or greater than the maximum human daily dose (MHD) on a mg/m2 basis) and 150 mg/kg/day or greater in dogs (approximately 1.4 times the MHD or greater on a mg/m2 basis). Fertility studies in rats have shown doses up to 350 mg/kg/day (approximately equal to the MHD on a mg/m2 basis) for 60 days to have no effect on fertility. The effect of valproate on testicular development and on sperm production and fertility in humans is unknown.
CLINICAL STUDIES
14.1Mania
The effectiveness of divalproex sodium extended-release tablets for the treatment of acute mania is based in part on studies establishing the effectiveness of divalproex sodium delayed release tablets for this indication. Divalproex sodium extended-release tablets effectiveness was confirmed in one randomized, double-blind, placebo-controlled, parallel group, 3-week, multicenter study. The study was designed to evaluate the safety and efficacy of divalproex sodium extended-release tablets in the treatment of bipolar I disorder, manic or mixed type, in adults. Adult male and female patients who had a current DSM-IV TR primary diagnosis of bipolar I disorder, manic or mixed type, and who were hospitalized for acute mania, were enrolled into this study. Divalproex sodium extended-release tablets was initiated at a dose of 25 mg/kg/day given once daily, increased by 500 mg/day on Day 3, then adjusted to achieve plasma valproate concentrations in the range of 85-125 mcg/mL. Mean daily divalproex sodium delayed-release tablets doses for observed cases were 2362 mg (range: 500-4000), 2874 mg (range: 1500-4500), 2993 mg (range: 1500-4500), 3181 mg (range: 1500-5000), and 3353 mg (range: 1500-5500) at Days 1, 5, 10, 15, and 21, respectively. Mean valproate concentrations were 96.5 mcg/mL, 102.1 mcg/mL, 98.5 mcg/mL, 89.5 mcg/mL at Days 5, 10, 15 and 21, respectively. Patients were assessed on the Mania Rating Scale (MRS; score ranges from 0-52).
Divalproex sodium extended-release tablets was significantly more effective than placebo in reduction of the MRS total score.
14.2Epilepsy
The efficacy of valproate in reducing the incidence of complex partial seizures (CPS) that occur in isolation or in association with other seizure types was established in two controlled trials. In one, multiclinic, placebo controlled study employing an add-on design, (adjunctive therapy) 144 patients who continued to suffer eight or more CPS per 8 weeks during an 8 week period of monotherapy with doses of either carbamazepine or phenytoin sufficient to assure plasma concentrations within the “therapeutic range” were randomized to receive, in addition to their original antiepilepsy drug (AED), either divalproex sodium delayed-release tablets or placebo. Randomized patients were to be followed for a total of 16 weeks. The following Table presents the findings.
| Add on
Treatment | Number of
Patients | Baseline
Incidence | Experimental
Incidence |
|---|---|---|---|
| Divalproex sodium delayed-release tablets | 75 | 16.0 | 8.9* |
| Placebo | 69 | 14.5 | 11.5 |
*Reduction from baseline statistically significantly greater for valproate than placebo at p ≤ 0.05 level.
Figure 1 presents the proportion of patients (X axis) whose percentage reduction from baseline in complex partial seizure rates was at least as great as that indicated on the Y axis in the adjunctive therapy study. A positive percent reduction indicates an improvement (i.e., a decrease in seizure frequency), while a negative percent reduction indicates worsening. Thus, in a display of this type, the curve for an effective treatment is shifted to the left of the curve for placebo. This Figure shows that the proportion of patients achieving any particular level of improvement was consistently higher for valproate than for placebo. For example, 45% of patients treated with valproate had a ≥ 50% reduction in complex partial seizure rate compared to 23% of patients treated with placebo.
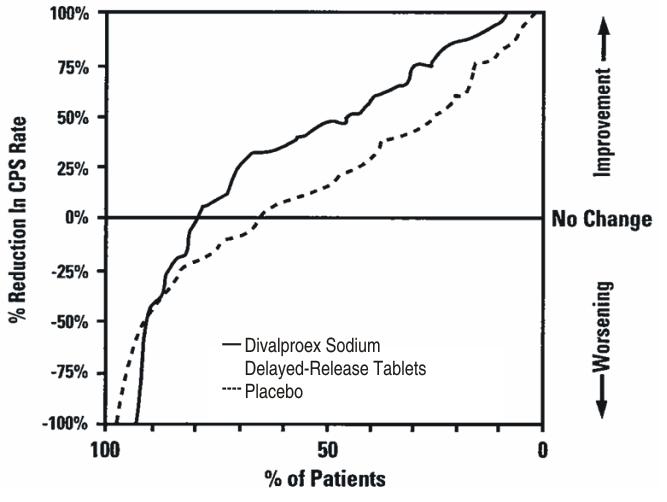 The second study assessed the capacity of valproate to reduce the incidence of CPS when administered as the sole AED. The study compared the incidence of CPS among patients randomized to either a high or low dose treatment arm. Patients qualified for entry into the randomized comparison phase of this study only if 1) they continued to experience 2 or more CPS per 4 weeks during an 8 to 12 week long period of monotherapy with adequate doses of an AED (i.e., phenytoin, carbamazepine, phenobarbital, or primidone) and 2) they made a successful transition over a two week interval to valproate. Patients entering the randomized phase were then brought to their assigned target dose, gradually tapered off their concomitant AED and followed for an interval as long as 22 weeks. Less than 50% of the patients randomized, however, completed the study. In patients converted to divalproex sodium delayed-release tablets monotherapy, the mean total valproate concentrations during monotherapy were 71 and 123 mcg/mL in the low dose and high dose groups, respectively.
The second study assessed the capacity of valproate to reduce the incidence of CPS when administered as the sole AED. The study compared the incidence of CPS among patients randomized to either a high or low dose treatment arm. Patients qualified for entry into the randomized comparison phase of this study only if 1) they continued to experience 2 or more CPS per 4 weeks during an 8 to 12 week long period of monotherapy with adequate doses of an AED (i.e., phenytoin, carbamazepine, phenobarbital, or primidone) and 2) they made a successful transition over a two week interval to valproate. Patients entering the randomized phase were then brought to their assigned target dose, gradually tapered off their concomitant AED and followed for an interval as long as 22 weeks. Less than 50% of the patients randomized, however, completed the study. In patients converted to divalproex sodium delayed-release tablets monotherapy, the mean total valproate concentrations during monotherapy were 71 and 123 mcg/mL in the low dose and high dose groups, respectively.
The following Table presents the findings for all patients randomized who had at least one post-randomization assessment.
| Treatment | Number of Patients | Baseline
Incidence | Randomized Phase
Incidence |
|---|---|---|---|
| High dose Valproate | 131 | 13.2 | 10.7* |
| Low dose Valproate | 134 | 14.2 | 13.8 |
*Reduction from baseline statistically significantly greater for high dose than low dose at p ≤ 0.05 level.
Figure 2 presents the proportion of patients (X axis) whose percentage reduction from baseline in complex partial seizure rates was at least as great as that indicated on the Y axis in the monotherapy study. A positive percent reduction indicates an improvement (i.e., a decrease in seizure frequency), while a negative percent reduction indicates worsening. Thus, in a display of this type, the curve for a more effective treatment is shifted to the left of the curve for a less effective treatment. This Figure shows that the proportion of patients achieving any particular level of reduction was consistently higher for high dose valproate than for low dose valproate. For example, when switching from carbamazepine, phenytoin, phenobarbital or primidone monotherapy to high dose valproate monotherapy, 63% of patients experienced no change or a reduction in complex partial seizure rates compared to 54% of patients receiving low dose valproate.
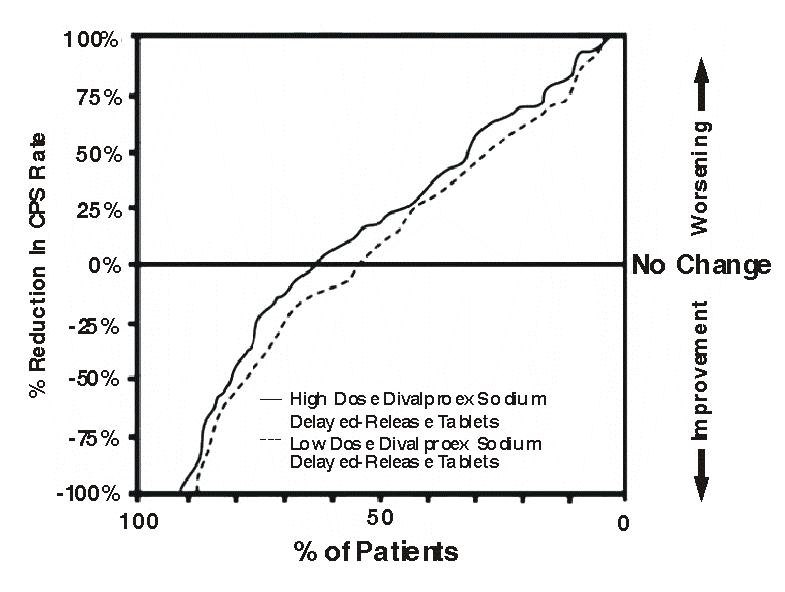
Information on pediatric studies are presented in section 8.
14.3Migraine
The results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group clinical trial demonstrated the effectiveness of divalproex sodium extended-release tablets in the prophylactic treatment of migraine headache. This trial recruited patients with a history of migraine headaches with or without aura occurring on average twice or more a month for the preceding three months. Patients with cluster or chronic daily headaches were excluded. Women of childbearing potential were allowed in the trial if they were deemed to be practicing an effective method of contraception.
Patients who experienced ≥ 2 migraine headaches in the 4-week baseline period were randomized in a 1:1 ratio to divalproex sodium extended-release tablets or placebo and treated for 12 weeks. Patients initiated treatment on 500 mg once daily for one week, and were then increased to 1000 mg once daily with an option to permanently decrease the dose back to 500 mg once daily during the second week of treatment if intolerance occurred. Ninety-eight of 114 divalproex sodium extended-release-treated patients (86%) and 100 of 110 placebo-treated patients (91%) treated at least two weeks maintained the 1000 mg once daily dose for the duration of their treatment periods. Treatment outcome was assessed on the basis of reduction in 4-week migraine headache rate in the treatment period compared to the baseline period. Patients (50 male, 187 female) ranging in age from 16 to 69 were treated with divalproex sodium extended release tablets (N=122) or placebo (N=115). Four patients were below the age of 18 and 3 were above the age of 65. Two hundred and two patients (101 in each treatment group) completed the treatment period. The mean reduction in 4-week migraine headache rate was 1.2 from a baseline mean of 4.4 in the divalproex sodium extended release tablets group, versus 0.6 from a baseline mean of 4.2 in the placebo group. The treatment difference was statistically significant (see Figure 3).
Figure 3. Mean Reduction In 4-Week Migraine Headache Rates
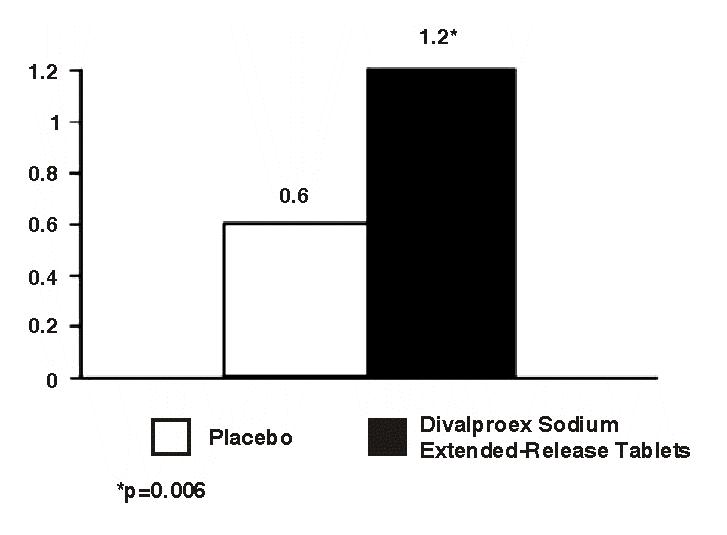
16HOW SUPPLIED/STORAGE AND HANDLING
Divalproex sodium extended-release 250 mg tablets are available as white, oval shaped film coated biconvex beveled edge tablets, debossed with W on one side and 724 on other side. Each divalproex sodium extended-release tablet contains divalproex sodium equivalent to 250 mg of valproic acid in the following packaging sizes:
Bottles of 30 (NDC 64679-724-01)
Bottles of 100 (NDC 64679-724-02)
Bottles of 500 (NDC 64679-724-03)
Unit Dose Pack
of 100 (10 x 10) (NDC 64679-724-04)
Divalproex sodium extended-release 500 mg tablets are available as dark grey colored, oval shaped film coated biconvex tablets, debossed with W725 on one side and plain on other side. Each divalproex sodium extended-release tablet contains divalproex sodium equivalent to 500 mg of valproic acid in the following packaging sizes:
Bottles of 30 (NDC 64679-725-01)
Bottles of 100 (NDC 64679-725-02)
Bottles of 500 (NDC 64679-725-03)
Unit Dose Pack
of 100 (10x10) (NDC 64679-725-04)
Recommended Storage
Store at 20
0-25
0C (68
0-77
0F) [see USP Controlled Room Temperature
17PATIENT COUNSELING INFORMATION
17.1Hepatotoxicity
Patients and guardians should be warned that nausea, vomiting, abdominal pain, anorexia, diarrhea, asthenia, and/or jaundice can be symptoms of hepatotoxicity and, therefore, require further medical evaluation promptly [see Warnings and Precautions (5.1)].
17.2Pancreatitis
Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis and, therefore, require further medical evaluation promptly [see Warnings and Precautions (5.3)].
17.3Teratogenicity/Usage in Pregnancy
Use of valproate during pregnancy increases the risk for neural tube defects and other malformations. Female patients of childbearing age, who require therapy for epilepsy, bipolar disorder, or migraines, should be advised of the risks of valproate use during pregnancy and appropriate therapeutic options. This is particularly important when the treatment of a spontaneously reversible condition not ordinarily associated with permanent injury or risk of death (e.g. migraine) is considered. Patients should read the Patient Information Leaflet, which appears as the last section of the labeling [see Use in Specific Populations (8.1)].
Patients should be encouraged to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 [see Use in Specific Populations (8.1)].
17.4Suicidal Thinking and Behavior
Patients, their caregivers, and families should be counseled that AEDs, including divalproex sodium extended-release tablets, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to the healthcare providers [see Warnings and Precautions (5.5)].
17.5Hyperammonemia
Patients should be informed of the signs and symptoms associated with hyperammonemic encephalopathy and be told to inform the prescriber if any of these symptoms occur [see Warnings and Precautions (5.7, 5.8)].
17.6CNS depression
Since valproate products may produce CNS depression, especially when combined with another CNS depressant (eg, alcohol), patients should be advised not to engage in hazardous activities, such as driving an automobile or operating dangerous machinery, until it is known that they do not become drowsy from the drug.
17.7Multi-organ Hypersensitivity Reaction
Patients should be instructed that a fever associated with other organ system involvement (rash, lymphadenopathy, etc.) may be drug-related and should be reported to the physician immediately [see Warnings and Precautions (5.10)].
17.8 FDA-Approved Patient Labeling
Important Information for Women Who Could Become Pregnant About the Use of divalproex sodium extended-release tablets.
Please read this leaflet carefully before you take any of this medication. This leaflet provides a summary of important information about taking this medication to women who could become pregnant. If you have any questions or concerns, or want more information about this medication, contact your doctor or pharmacist.
Information For Women Who Could Become Pregnant
You can only obtain this medication by prescription from your doctor. The decision to use this medicine should be made by you and your doctor based on your health needs and medical condition.
Before starting this medicine, you should know that using this medicine during pregnancy causes an increased chance of birth defects in your baby. These birth defects may include spina bifida and other defects where the spinal canal does not close normally. These defects usually occur in 1 to 2 out of every 1000 babies born in the United States. Studies show that for babies born to epileptic women who took valproate in the first 12 weeks of pregnancy, these defects occur in 1 to 2 out of every 100 babies.
Use of valproate during pregnancy also increases the chance of other birth defects such as of the heart, bones, and other parts of the body. Studies suggest that other medicines used to treat your condition may be less likely to cause these defects.
Information For Women Who Are Planning to Get Pregnant
Women using valproate who plan to get pregnant should discuss their treatment options with their doctor.
Information For Women Who Become Pregnant
If you become pregnant while taking valproate, you should contact your doctor immediately.
Other Important Information
- You should take your medicine exactly as prescribed by your doctor to get the most benefit from your medicine and reduce the risk of side effects.
- If you have taken more than the prescribed dose, contact your hospital emergency room or local poison center immediately.
- Your medicine was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
Facts About Birth Defects
It is important to know that birth defects may occur even in children born to women who are not taking any medicines and do not have other risk factors.
This summary provides important information about the use of divalproex sodium extended-release tablets to women who could become pregnant. If you would like more information, ask your doctor or pharmacist to let you read the professional labeling and then discuss it with them. If you have any questions or concerns about taking this medication, you should discuss them with your doctor.
Manufactured by:
Wockhardt Limited,
Mumbai, India.
Distributed by:
Wockhardt USA LLC.
20 Waterview Blvd.
Parsippany, NJ 07054,
USA.
DRUG: DIVALPROEX SODIUMER ER
GENERIC: DIVALPROEX SODIUM
DOSAGE: TABLET, FILM COATED, EXTENDED RELEASE
ADMINSTRATION: ORAL
NDC: 49349-411-02
COLOR: white
SHAPE: OVAL
SCORE: No score
SIZE: 15 mm
IMPRINT: W;724
PACKAGING: 30 in 1 BLISTER PACK
ACTIVE INGREDIENT(S):
- DIVALPROEX SODIUM 250mg in 1
INACTIVE INGREDIENT(S):

| DIVALPROEX SODIUM
divalproex sodium tablet, film coated, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |