Label: DILTIAZEM HYDROCHLORIDE injection, solution
- NDC Code(s): 51754-3000-3, 51754-3500-3
- Packager: Exela Pharma Sciences,LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 30, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DILTIAZEM HYDROCHLORIDE IN DEXTROSE INJECTION safely and effectively. See full prescribing information for DILTIAZEM HYDROCHLORIDE IN DEXTROSE INJECTION.
DILTIAZEM HYDROCHLORIDE IN DEXTROSE injection, for intravenous use only
Initial U.S. Approval: 1982INDICATIONS AND USAGE
Diltiazem Hydrochloride in Dextrose Injection is a non-dihydropyridine calcium-channel blocker indicated for the following:
DOSAGE AND ADMINISTRATION
Continuous Intravenous Infusion
- •
- Immediately following bolus administration of 20 mg (0.25 mg/kg) or 25 mg (0.35 mg/kg) diltiazem hydrochloride injection and reduction of heart rate, begin an intravenous infusion. (2.1)
- •
- Initial infusion rate of diltiazem hydrochloride is 10 mg/h, rate may be increased in 5 mg/h increments up to 15 mg/h as needed if further reduction in heart rate is required. The infusion may be maintained for up to 24 hours. (2.1)
It is recommended that diltiazem hydrochloride not be mixed with any other drugs in the same container. If possible, it is recommended that diltiazem hydrochloride not be co-infused in the same intravenous line. (2.2)
DOSAGE FORMS AND STRENGTHS
Injection: Clear, colorless solution in single-dose bag for intravenous use. (3)
125 mg/ 125 mL (1 mg/mL) and 250 mg/250 mL (1 mg/mL)
CONTRAINDICATIONS
- •
- Sick sinus syndrome or second- or third-degree AV block with no functioning ventricular pacemaker. (4)
- •
- Severe hypotension or cardiogenic shock. (4)
- •
- Demonstrated hypersensitivity to the drug. (4)
- •
- Atrial fibrillation or atrial flutter associated with an accessory bypass tract such as in WPW syndrome or short PR syndrome. (4)
- •
- Ventricular tachycardia. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions are hypotension, itching and burning at injection site, vasodilation, and arrhythmia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Exela Pharma Sciences, LLC at 1-888-451-4321, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Anesthetics: Potential to cause depression of cardiac contractility, conductivity, and automaticity as well as the vascular dilation (7)
- •
- Benzodiazepines: Coadministration can result in increased clinical effects (e.g. prolonged sedation). (7)
- •
- Beta-Blockers: Monitor patients for bradycardia, AV block, and/or depression of contractility. (7)
- •
- Buipirone: Enhanced effects and increased toxicity of buspirone may be possible during concomitant administration with diltiazem. (7)
- •
- Carbamazepine: Patients should be closely monitored for carbamazepine toxicity. (7)
- •
- Cimetidine: Patients currently receiving diltiazem therapy should be carefully monitored for a change in pharmacological effect when initiating and discontinuing therapy with cimetidine. (7)
- •
- Clonidine: Monitor heart rate in patients receiving concomitant diltiazem and clonidine as bradycardia has been reported. (7)
- •
- Cyclosporine: Cyclosporine concentrations should be monitored, as pharmacokinetic interactions have been observed, especially when diltiazem therapy is initiated, adjusted, or discontinued. (7)
- •
- Digitalis: Monitor patients during concomitant administration. (7)
- •
- Ivabradine: Avoid concomitant use of ivabradine and diltiazem in patients experiencing bradycardia. (7)
- •
- Quinidine: Monitoring for quinidine adverse effects and adjust the dose accordingly. (7)
- •
- Rifampin: Coadministration of rifampin with diltiazem lowered the diltiazem plasma concentrations, avoid concomitant use when possible. (7)
- •
- Statins: The risk of myopathy and rhabdomyolysis with statins metabolized by CYP3A4 may be increased with concomitant use of diltiazem. When possible, use a non-CYP3A4-metabolized statin together with diltiazem; otherwise, dose adjustments for both diltiazem and the statin should be considered along with close monitoring for signs and symptoms of any statin related adverse events. (7)
USE IN SPECIFIC POPULATIONS
Lactation: Diltiazem is excreted into human milk. (8.2)
Revised: 11/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Atrial Fibrillation or Atrial Flutter
1.2 Paroxysmal Supraventricular Tachycardia
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
2.2 Intravenous Single Injections (Bolus)
2.3 Continuous Intravenous Infusion
2.4 Physical Incompatibilities
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hemodynamic Deterioration in Patients with Wide Complex Tachycardia
5.2 AV Block
5.3 Heart Failure
5.4 Hypotension
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
Administer intravenous diltiazem in a setting with continuous monitoring of the ECG and frequent measurement of blood pressure. A defibrillator and emergency equipment should be readily available.
2.2 Intravenous Single Injections (Bolus)
Withdraw the bolus volume from the diltiazem hydrochloride infusion bag and administer it over 2 minutes. The initial dose of diltiazem hydrochloride should be 0.25 mg/kg actual body weight as a bolus administered over 2 minutes (20 mg is a reasonable dose for the average patient). If response is inadequate, a second dose may be administered after 15 minutes. The second bolus dose of diltiazem hydrochloride should be 0.35 mg/kg actual body weight administered over 2 minutes (25 mg is a reasonable dose for the average patient). Subsequent intravenous bolus doses should be individualized for each patient. Patients with low body weight should be dosed on a mg/kg basis.
2.3 Continuous Intravenous Infusion
Immediately following bolus, the recommended initial infusion rate of diltiazem hydrochloride is 5 mg/hour. Adjust the infusion rate in 5 mg/hour increments up to a maximum of 15 mg/hour as needed to achieve satisfactory rate control. Infusions longer than 24 hours have not been studied. Patients should generally be transitioned to other antiarrhythmic agents within 24 hours [see Clinical Studies (14)].
2.4 Physical Incompatibilities
Do not mix diltiazem hydrochloride with any other drugs in the same container. If possible, use a dedicated line for diltiazem hydrochloride.
Diltiazem Hydrochloride has demonstrated physical incompatibilities with acetazolamide, acyclovir, aminophylline, ampicillin, ampicillin sodium/sulbactam sodium, cefamandole, cefoperazone, diazepam, furosemide, hydrocortisone sodium succinate, insulin (regular: 100 units/mL), methylprednisolone sodium succinate, mezlocillin, nafcillin, phenytoin, rifampin, and sodium bicarbonate.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Diltiazem Hydrochloride Injection is contraindicated in:
- •
- Patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker.
- •
- Patients with second- or third-degree AV block except in the presence of a functioning ventricular pacemaker.
- •
- Patients with severe hypotension or cardiogenic shock.
- •
- Patients who have demonstrated hypersensitivity to the drug.
- •
- Intravenous diltiazem and intravenous beta-blockers should not be administered together or in close proximity (within a few hours).
- •
- Patients with atrial fibrillation or atrial flutter associated with an accessory bypass tract such as in WPW syndrome or short PR syndrome.
As with other agents which slow AV nodal conduction and do not prolong the refractoriness of the accessory pathway (e.g., verapamil, digoxin), in rare instances patients in atrial fibrillation or atrial flutter associated with an accessory bypass tract may experience a potentially life-threatening increase in heart rate accompanied by hypotension when treated with diltiazem hydrochloride injection. As such, the initial use of diltiazem hydrochloride injection should be, if possible, in a setting where monitoring and resuscitation capabilities, including DC cardioversion/defibrillation, are present [see Overdosage (10)]. Once familiarity of the patient's response is established, use in an office setting may be acceptable. - •
- Patients with ventricular tachycardia. Administration of other calcium channel blockers to patients with wide complex tachycardia (QRS ≥ 0.12 seconds) has resulted in hemodynamic deterioration and ventricular fibrillation. It is important that an accurate pretreatment diagnosis distinguish wide complex QRS tachycardia of supraventricular origin from that of ventricular origin prior to administration of diltiazem hydrochloride injection.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hemodynamic Deterioration in Patients with Wide Complex Tachycardia
Diltiazem may cause hemodynamic deterioration and ventricular fibrillation if administered to patients with wide complex tachycardia of ventricular origin. Distinguish wide complex QRS tachycardia of supraventricular origin from that of ventricular origin prior to diltiazem administration [see Contraindications (4)].
5.2 AV Block
Diltiazem prolongs AV nodal conduction and refractoriness that may cause second- or third-degree AV block in sinus rhythm. Concomitant use of diltiazem with agents known to affect cardiac conduction may result in additive effects [see Drug Interactions (7)]. If high-degree AV block occurs in sinus rhythm, discontinue diltiazem and institute appropriate supportive measures [see Overdosage (10)].
5.3 Heart Failure
Diltiazem is a negative inotrope and can cause decreased systolic function and heart failure. Do not initiate in patients with acute decompensated heart failure or cardiogenic shock. If heart failure develops during diltiazem treatment, discontinue treatment and treat heart failure appropriately.
-
6 ADVERSE REACTIONS
The following adverse reaction rates are based on the use of diltiazem hydrochloride injection in over 400 domestic clinical trial patients with atrial fibrillation/flutter or PSVT under double-blind or open-label conditions.
Hypotension was the most commonly reported adverse event during clinical trials, with symptomatic hypotension occurring in 3.2% of patients. Other events reported in a least 1% of the diltiazem-treated patients were injection site reactions (e.g., itching, burning) - 3.9%, vasodilation (flushing) - 1.7%, and arrhythmia (junctional rhythm or isorhythmic dissociation) - 1%.
In addition, the following events were reported infrequently (less than 1%):
Cardiovascular - Asystole, atrial flutter, AV block first degree, AV block second degree, bradycardia, chest pain, congestive heart failure, sinus pause, sinus node dysfunction, syncope, ventricular arrhythmia, ventricular fibrillation, ventricular tachycardia
Dermatologic - Pruritus, sweating
Gastrointestinal - Constipation, elevated SGOT or alkaline phosphatase, nausea, vomiting
Nervous System - Dizziness, paresthesia
Other - Amblyopia, asthenia, dry mouth, dyspnea, edema, headache,
Although not observed in clinical trials with diltiazem hydrochloride injection, the following events associated with oral diltiazem may occur:
Dermatologic - Erythema multiforme (including Stevens-Johnson syndrome, toxic epidermal necrolysis), exfoliative dermatitis, leukocytoclastic vasculitis, petechiae, photosensitivity, purpura, rash, urticaria
Gastrointestinal - Anorexia, diarrhea, dysgeusia, dyspepsia, mild elevations of SGPT and LDH, weight increase
Other - Allergic reactions, angioedema (including facial or periorbital edema), CPK elevation, gingival hyperplasia, hemolytic anemia, hyperglycemia, leukopenia, muscle cramps, myopathy, nasal congestion
-
7 DRUG INTERACTIONS
Table 1. Clinically Relevant Interactions with Diltiazem
Drug/Substance Class or Name
Clinical implication
Prevention/Management
Agents Known to decrease peripheral resistance, cardiac contractility or conduction
Increased risk of bradycardia, AV block, and heart failure.
Monitor.
Beta-blockers
Increased risk of bradycardia, AV block, and depression of contractility.
Beta-blocker may need to be decreased [see Warnings and Precautions (5.1, 5.2)].
Anesthetics
Depressed cardiac contractility, conductivity, and automaticity as well as the vascular dilation.
Monitor.
Clonidine
Increased risk of bradycardia.
Monitor.
Drugs metabolized by Cytochrome P450 3A4
Diltiazem is both a substrate and an inhibitor of the cytochrome P450 3A4 enzyme system. Concomitant use with diltiazem can increase exposure of drugs that are substrates of CYP450 3A4.
Drugs that are substrates of CYP450 3A4 may require dose adjustment to maintain optimum therapeutic blood levels.
Benzodiazepines
Increased exposure likely.
Monitor.
Buspirone
Carbamazepine
Cyclosporine
Quinidine
Ranolazine
Limit ranolazine to 500 mg twice daily.
Statins
Increased risk of myopathy and rhabdomyolysis with statins metabolized by CYP3A4.
When possible, use a non-CYP3A4-metabolized statin with diltiazem.
Limit daily dose of simvastatin to 10 mg and diltiazem to 240 mg [see Clinical Pharmacology (12.3)].
Ivabradine
May exacerbate bradycardia and conduction disturbances.
Avoid concomitant use [see Clinical Pharmacology (12.3)].
Effect of other drugs on Diltiazem
Cytochrome P450 3A4 Inhibitors/ Inducers
Diltiazem is a substrate of the cytochrome P450 3A4 enzyme and inhibitors/inducers may change the pharmacological effect of diltiazem
Monitor.
Strong or moderate CYP 3A inhibitors a
Examples b: Ketoconazole, itraconazole, cimetidine
Patients currently receiving diltiazem therapy should be carefully monitored for a change in pharmacological effect when initiating and discontinuing therapy with strong or moderate inhibitors of CYP3A4.
An adjustment in the diltiazem dose may be warranted
CYP inducers
Examples b: Rifampin, carbamazepine
CYP3A inducers can lower the plasma levels of diltiazem.
Coadministration of diltiazem with rifampin or any known CYP3A4 inducer should be avoided when possible, and alternative therapy considered [see Clinical Pharmacology (12.3)].
- a Strong and moderate inhibitors increase the AUC of sensitive substrates of CYP3A4 by ≥ 5 and ≥ 2-fold, respectively
- b These examples are a guide and not considered a comprehensive list of all possible drugs that may fit this category. The healthcare provider should consult appropriate references for comprehensive information.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available data from the published literature over decades of use with diltiazem during pregnancy have not identified a drug associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, decreased embryo and fetal survival rates, and skeletal abnormalities have been observed at oral doses five to ten times the human oral antianginal therapeutic dose, and reduction in pup weights was also observed. At 20 times the human oral antianginal therapeutic dose, an increase in stillbirths was observed (see Data).
The estimated background risk for major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Reproduction studies have been conducted in mice, rats, and rabbits. Administration of oral doses ranging from five to ten times greater (on a mg/kg basis) than the human oral antianginal therapeutic dose has resulted in embryo and fetal lethality. These doses, in some studies, have been reported to cause skeletal abnormalities. In the perinatal/postnatal studies there was some reduction in early individual pup weights and survival rates. There was an increased incidence of stillbirths at doses of 20 times the human oral antianginal therapeutic dose or greater.
8.2 Lactation
Risk Summary
Published literature reports the presence of diltiazem in human milk. One report with oral diltiazem suggests that concentrations in breast milk may approximate serum levels. There are no data on the effects of diltiazem on the breastfed child or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Diltiazem Hydrochloride Injection and any potential adverse effects on the breastfed child from Diltiazem Hydrochloride Injection or from the underlying maternal condition.
8.5 Geriatric Use
Clinical studies of diltiazem did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Overdosage experience is limited. In the event of overdosage or an exaggerated response, appropriate supportive measures should be employed.
Diltiazem does not appear to be removed by peritoneal or hemodialysis. Limited data suggest that plasmapheresis may hasten diltiazem elimination following overdose.
The intravenous LD50's in mice and rats were 60 and 38 mg/kg, respectively. The toxic dose in humans is not known.
-
11 DESCRIPTION
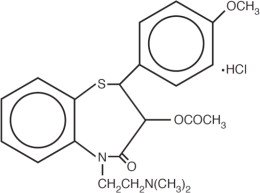
Diltiazem Hydrochloride Injection is a calcium ion influx inhibitor (slow channel blocker or calcium channel antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)one,3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2, 3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride, (+)-cis-. The structural formula is:
Diltiazem hydrochloride is a white to off-white crystalline powder with a bitter taste soluble in water, methanol, and chloroform.
Diltiazem Hydrochloride Injection is a clear, colorless, sterile, nonpyrogenic, isotonic solution for intravenous use only. Each mL contains 1 mg diltiazem hydrochloride, USP, 50 mg anhydrous dextrose, USP, 0.15 mg citric acid monohydrate, USP and 0.12 mg sodium citrate dihydrate, USP, sodium hydroxide, NF and/or hydrochloric Acid, NF to adjust pH to 3.9, and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The therapeutic effects of diltiazem are believed to be related to inhibiting influx of calcium ions during membrane depolarization of cardiac and vascular smooth muscle.
Paroxysmal Supraventricular Tachycardia: Diltiazem slows AV nodal conduction time and prolongs AV nodal refractoriness.Diltiazem exhibits frequency- (use-) dependent effects on AV nodal conduction such that it may selectively reduce the heart rate during tachycardias involving the AV node with little or no effect on normal AV nodal conduction at normal heart rates.
Atrial Fibrillation or Atrial Flutter: Diltiazem slows the ventricular rate in patients with a rapid ventricular response during atrial fibrillation or atrial flutter. Diltiazem converts paroxysmal supraventricular tachycardia (PSVT) to normal sinus rhythm by interrupting the reentry circuit in AV nodal reentrant tachycardias and reciprocating tachycardias, e.g., Wolff- Parkinson-White syndrome (WPW).
Diltiazem prolongs the sinus cycle length. It has no effect on the sinus node recovery time or on the sinoatrial conduction time in patients without SA nodal dysfunction. Diltiazem has no significant electrophysiologic effect on tissues in the heart that are fast sodium channel dependent, e.g., His-Purkinje tissue, atrial and ventricular muscle, and extra nodal accessory pathways.
Like other calcium channel antagonists, because of its effect on vascular smooth muscle, diltiazem decreases total peripheral resistance resulting in a decrease in both systolic and diastolic blood pressure.
12.2 Pharmacodynamics
Intravenous diltiazem hydrochloride 20 mg prolongs AH conduction time and AV node functional and effective refractory periods by approximately 20%. PR in healthy volunteers and HR in patients with atrial fibrillation and atrial flutter are dependent on plasma level of diltiazem. Based on this relationship, the mean plasma diltiazem concentration required to produce a 20%, 30% and 40% decrease in heart rate was determined to be 80 ng/mL, 130 ng/mL and 300 ng/mL, respectively.
In patients with cardiovascular disease, diltiazem hydrochloride administered intravenously in single bolus doses, followed in some cases by a continuous infusion, reduced blood pressure, systemic vascular resistance, the rate-pressure product, and coronary vascular resistance and increased coronary blood flow. In a limited number of studies of patients with compromised myocardium (severe congestive heart failure, acute myocardial infarction, hypertrophic cardiomyopathy), administration of intravenous diltiazem produced no significant effect on contractility, left ventricular end diastolic pressure, or pulmonary capillary wedge pressure. The mean ejection fraction and cardiac output/index remained unchanged or increased. Maximal hemodynamic effects usually occurred within 2 to 5 minutes of an injection. However, in rare instances, worsening of congestive heart failure has been reported in patients with preexisting impaired ventricular function.
12.3 Pharmacokinetics
Based on the results of pharmacokinetic studies in healthy volunteers administered different oral diltiazem hydrochloride formulations, constant rate intravenous infusions of diltiazem hydrochloride at 3, 5, 7, and 11 mg/h are predicted to produce steady-state plasma diltiazem concentrations equivalent to 120-, 180-, 240-, and 360-mg total daily oral doses of diltiazem hydrochloride tablets and diltiazem hydrochloride extended-release capsules.
Distribution
The volume of distribution of diltiazem is approximately 305 L. Diltiazem is 70% to 80% bound to plasma proteins. In vitro studies suggest alpha1-acid glycoprotein binds approximately 40% of the drug at clinically significant concentrations. Albumin appears to bind approximately 30% of the drug, while other constituents bind the remaining bound fraction. Competitive in vitro ligand binding studies have shown that diltiazem hydrochloride binding is not altered by therapeutic concentrations of digoxin, phenytoin, hydrochlorothiazide, indomethacin, phenylbutazone, propranolol, salicylic acid, tolbutamide, or warfarin.
Metabolism and excretion
Diltiazem is extensively metabolized in the liver. After oral administration, diltiazem undergoes extensive metabolism in man by deacetylation, N-demethylation, and O-demethylation via cytochrome P-450 (oxidative metabolism) in addition to conjugation. Metabolites N-monodesmethyldiltiazem, desacetyldiltiazem, desacetyl-N-monodesmethyldiltiazem, desacetyl-O-desmethyldiltiazem, and desacetyl-N, O-desmethyldiltiazem have been identified in human urine. These metabolites are also observed following 24-hour constant rate intravenous infusion.
The systemic clearance of diltiazem has been found to be decreased in patients with atrial fibrillation or atrial flutter, compared to healthy volunteers. In patients administered continuous infusions at 10 mg/h or 15 mg/h for 24 h, diltiazem systemic clearance averaged 42 L/h and 31 L/h, respectively. The plasma elimination half-life is approximately 3.4 h. Total radioactivity measurement following short IV administration in healthy volunteers suggests the presence of other unidentified metabolites which attain higher concentrations than those of diltiazem and are more slowly eliminated; half-life of total radioactivity is about 20 h compared to 2 to 5 h for diltiazem.
After constant rate intravenous infusion to healthy male volunteers, diltiazem exhibits nonlinear pharmacokinetics over an infusion range of 4.8 to 13.2 mg/h for 24 h. Over this infusion range, as the dose is increased, systemic clearance decreases from 64 to 48 L/h while the plasma elimination half-life increases from 4.1 to 4.9 h. The volume of distribution remains unchanged (360 to 391 L).
Specific Populations
Renal insufficiency, or even end-stage renal disease, does not appear to influence diltiazem disposition following oral administration. Liver cirrhosis reduces diltiazem's apparent clearance and prolong its half-life.
Drug Interaction Studies
Effect of Diltiazem on Other Drugs:
Agents known to Decrease Peripheral Resistance, Cardiac Contractility and Conduction
Beta-blockers: Controlled and uncontrolled domestic studies suggest that concomitant use of diltiazem and beta-blockers is usually well tolerated, but available data are not sufficient to predict the effects of concomitant treatment in patients with left ventricular dysfunction or cardiac conduction abnormalities. Administration of diltiazem concomitantly with propranolol in five normal volunteers resulted in increased propranolol levels in all subjects and bioavailability of propranolol was increased approximately 50%. In vitro, propranolol appears to be displaced from its binding sites by diltiazem [see Warnings and Precautions (5.1, 5.2)].
Digitalis: Intravenous diltiazem has been administered to patients receiving either intravenous or oral digitalis therapy. The combination of the two drugs was well tolerated without serious adverse effects.
Ivabradine: Coadministration with diltiazem resulted in approximately 3-fold the AUC and Cmax of ivabradine and 20-60% increase in the active metabolite (S18982) exposure [see Drug Interactions (7)].
CYP3A4 Substrates
Benzodiazepines: With diltiazem, the AUC of midazolam and triazolam is 3- to 4-fold and the Cmax is 2-fold what they are alone. The elimination half-life of midazolam and triazolam also increased by 50-150% during coadministration with diltiazem [see Drug Interactions (7)].
Buspirone: With diltiazem, the mean buspirone AUC was about 5.5-fold and Cmax was about 4.1-fold what they are alone. The t1/2 and Tmax of buspirone were not affected by diltiazem [see Drug Interactions (7)].
Carbamazepine: Concomitant administration of diltiazem with carbamazepine was reported to result in elevated serum levels of carbamazepine (40% to 72% increase), resulting in toxicity in some cases [see Drug Interactions (7)].
Cyclosporine: In renal and cardiac transplant recipients, a reduction of cyclosporine dose ranging from 15% to 48% was necessary to maintain cyclosporine trough concentrations similar to those seen prior to the addition of diltiazem. If these agents are to be administered concurrently, cyclosporine concentrations should be monitored, especially when diltiazem therapy is initiated, adjusted, or discontinued. The effect of cyclosporine on diltiazem plasma concentrations has not been evaluated [see Drug Interactions (7)].
Quinidine: Diltiazem increases the AUC of quinidine by 51%, elimination half-life by 36%, and decreases its oral clearance by 33% [see Drug Interactions (7)].
Ranolazine: On coadministration with diltiazem 180 to 360 mg daily, the plasma levels of ranolazine are 2.2-to 2.8-fold what they are alone. Diltiazem plasma levels are not affected by ranolazine [see Drug Interactions (7)].
Statins: Diltiazem has been shown to increase the AUC of some statins. The risk of myopathy and rhabdomyolysis with statins metabolized by CYP3A4 may be increased with concomitant use of diltiazem [see Drug Interactions (7)].
Coadministration of a simvastatin with 120 mg BID diltiazem SR resulted in 5 times the mean simvastatin AUC versus simvastatin alone. Higher doses of diltiazem are likely to be worse.
Coadministration of a lovastatin with 120 mg BID diltiazem SR resulted in a 3 to 4 times the mean lovastatin AUC and Cmax versus lovastatin alone. In the same study, there was no significant change in AUC and Cmax of 20 mg single dose pravastatin during diltiazem coadministration. Diltiazem plasma levels were not significantly affected by lovastatin or pravastatin.
Effect of Other Drugs on Diltiazem:
CYP3A4 Inhibitors and Inducers
Cimetidine and Ranitidine: Coadministration with cimetidine increased Cmax of diltiazem by 58% and AUC by 53%. Ranitidine produced smaller, non-significant increases. The effect may be mediated by cimetidine's known inhibition of hepatic CYP3A, the enzyme system responsible for the first-pass metabolism of diltiazem [see Drug Interactions (7)].
Rifampin: Coadministration of rifampin with diltiazem lowered the diltiazem plasma concentrations to undetectable levels [see Drug Interactions (7)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 24-month study in rats at oral dosage levels of up to 100 mg/kg/day and a 21-month study in mice at oral dosage levels of up to 30 mg/kg/day showed no evidence of carcinogenicity. There was also no mutagenic response in vitro or in vivo in mammalian cell assays or in vitro in bacteria. No evidence of impaired fertility was observed in a study performed in male and female rats at oral dosages of up to 100 mg/kg/day.
-
14 CLINICAL STUDIES
In domestic controlled trials in patients with atrial fibrillation or atrial flutter, bolus administration of diltiazem hydrochloride injection was effective in reducing heart rate by at least 20% in 95% of patients. Diltiazem hydrochloride injection rarely converts atrial fibrillation or atrial flutter to normal sinus rhythm. Following administration of one or two intravenous bolus doses of diltiazem injection, response usually occurs within 3 minutes and maximal heart rate reduction generally occurs in 2 to 7 minutes. Heart rate reduction may last from 1 to 3 hours. If hypotension occurs, it is generally short-lived, but may last from 1 to 3 hours.
A 24-hour continuous infusion of diltiazem injection in the treatment of atrial fibrillation or atrial flutter maintained at least a 20% heart rate reduction during the infusion in 83% of patients. Upon discontinuation of infusion, heart rate reduction may last from 0.5 hours to more than 10 hours (median duration 7 hours). Hypotension, if it occurs, may be similarly persistent.
In the controlled clinical trials, 3.2% of patients required some form of intervention (typically, use of intravenous fluids or the Trendelenburg position) for blood pressure support following diltiazem hydrochloride injection.
In domestic controlled trials, bolus administration of diltiazem hydrochloride injection was effective in converting PSVT to normal sinus rhythm in 88% of patients within 3 minutes of the first or second bolus dose.
Symptoms associated with the arrhythmia were improved in conjunction with decreased heart rate or conversion to normal sinus rhythm following administration of diltiazem hydrochloride injection.
In controlled clinical trials, therapy with antiarrhythmic agents to maintain reduced heart rate in atrial fibrillation or atrial flutter or for prophylaxis of PSVT was generally started within 3 hours after bolus administration of diltiazem hydrochloride. These antiarrhythmic agents were intravenous or oral digoxin, Class 1 antiarrhythmics (e.g., quinidine, procainamide), calcium channel blockers, and oral beta-blockers.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Diltiazem Hydrochloride Injection is supplied as a sterile, unpreserved, colorless solution in a single-dose polymeric bag containing 1 mg/mL of diltiazem as either a 125 mg of diltiazem in 125 mL of solution (51754-3000-1) or 250 mg diltiazem in 250 mL (51754-3500-1) sealed with a Twist Off port and oversealed in an aluminum pouch.
Discard any unused portion.
Store under refrigeration at 2°C to 8°C (36°F to 46°F). The single-dose bags in their original pouches are stable for up to one month at room temperature without significant loss of potency. The bag and port is not made with natural rubber latex, PVC, or DEHP.
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-125 mL IV Bag Label
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-125 mL Overwrap Label
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-250 mL IV Bag Label
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-250 mL Overwrap Label
-
INGREDIENTS AND APPEARANCE
DILTIAZEM HYDROCHLORIDE
diltiazem hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51754-3000 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DILTIAZEM HYDROCHLORIDE (UNII: OLH94387TE) (DILTIAZEM - UNII:EE92BBP03H) DILTIAZEM HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) May contain HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51754-3000-3 10 in 1 CARTON 11/30/2021 1 125 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215252 11/30/2021 DILTIAZEM HYDROCHLORIDE
diltiazem hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51754-3500 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DILTIAZEM HYDROCHLORIDE (UNII: OLH94387TE) (DILTIAZEM - UNII:EE92BBP03H) DILTIAZEM HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) May contain HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51754-3500-3 10 in 1 CARTON 11/30/2021 1 250 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215252 11/30/2021 Labeler - Exela Pharma Sciences,LLC (831274399) Establishment Name Address ID/FEI Business Operations Exela Pharma Sceinces, LLC 831274399 ANALYSIS(51754-3000, 51754-3500) , MANUFACTURE(51754-3000, 51754-3500) , LABEL(51754-3000, 51754-3500) , PACK(51754-3000, 51754-3500)