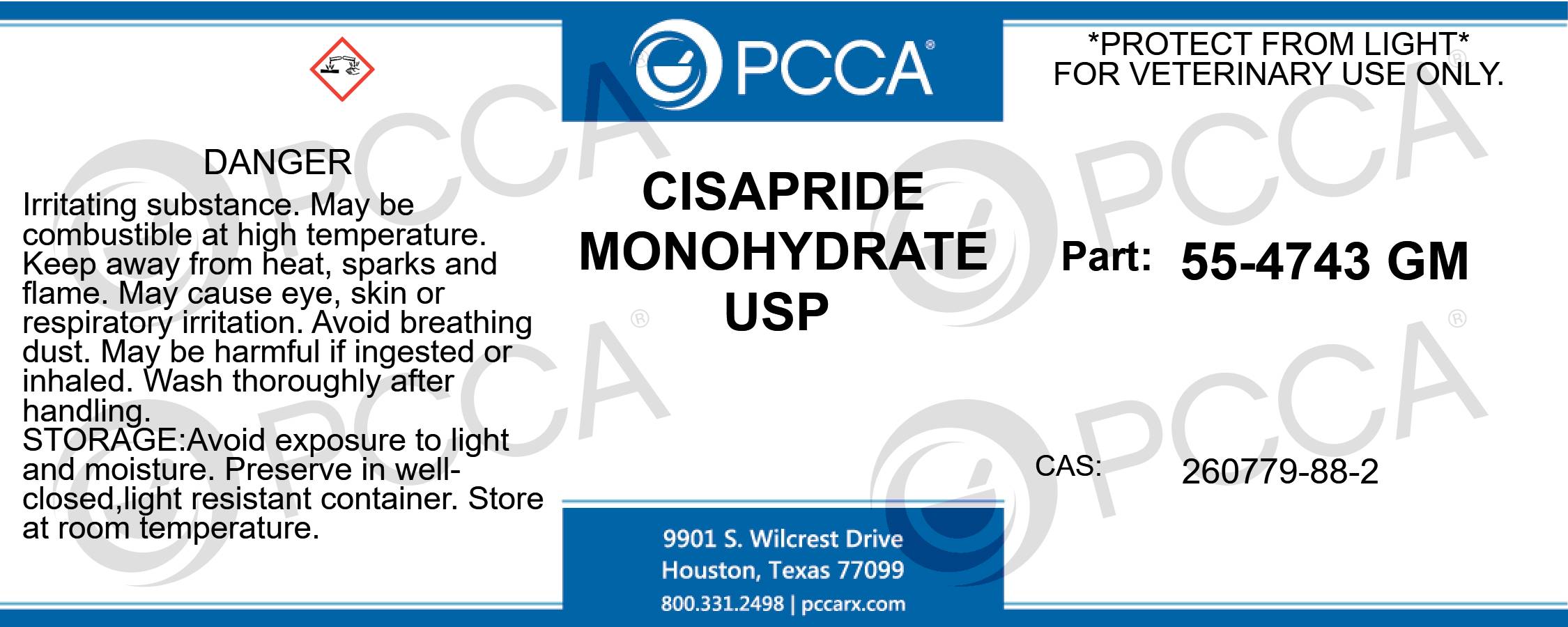
Label: CISAPRIDE MONOHYDRATE powder
- NDC Code(s): 51927-0257-0
- Packager: Professional Compounding Centers of America dba PCCA
- This is a repackaged label.
- Source NDC Code(s): 66577-023
- Category: BULK INGREDIENT - ANIMAL DRUG
- DEA Schedule: None
- Marketing Status: Bulk Ingredient For Animal Drug Compounding
Drug Label Information
Updated July 15, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL – BULK PRODUCT
CISAPRIDE MONOHYDRATE USP
*PROTECT FROM LIGHT*
FOR VETERINARY USE ONLY.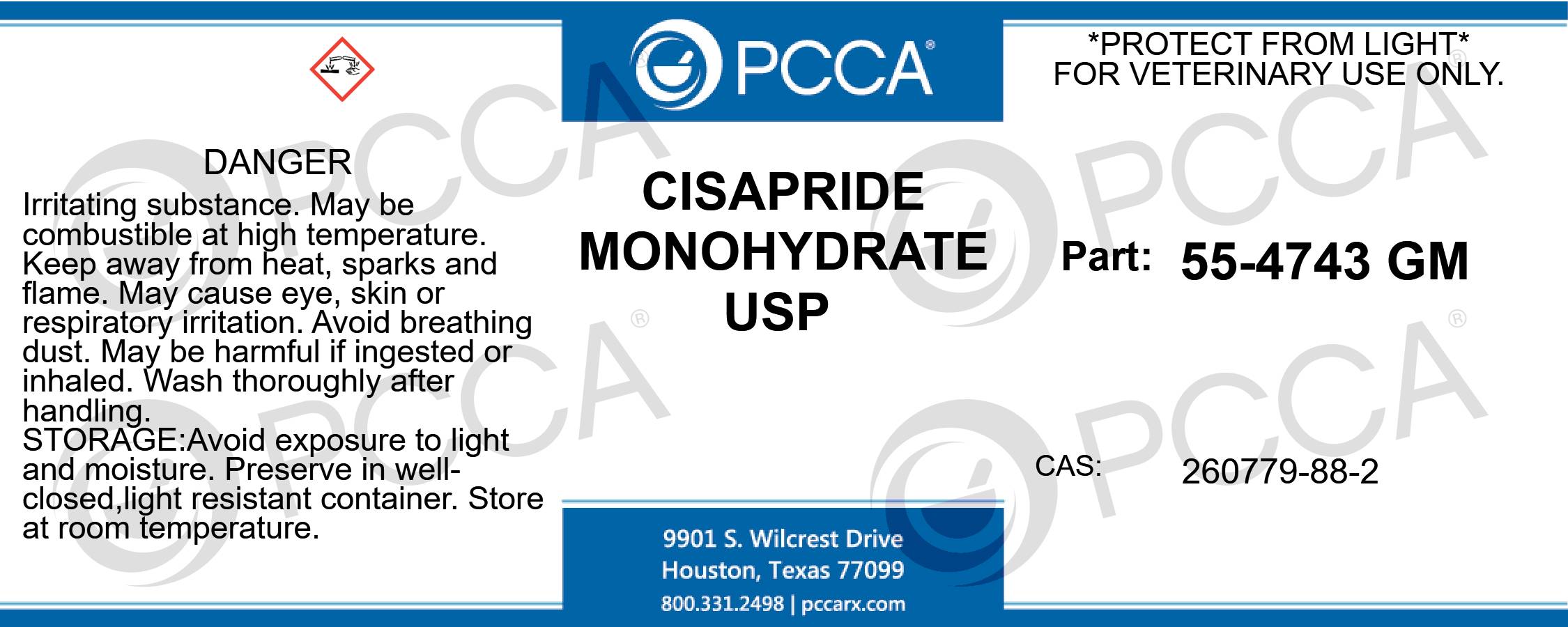
PART: 55-4743 GM
CAS: 260779-88-2
DANGER: IRRITATING SUBSTANCE. MAY BE COMBUSTIBLE AT HIGH TEMPERATURE. KEEP AWAY FROM HEAT, SPARKS AND FLAME. MAY CAUSE EYE, SKIN OR RESPIRATORY IRRITATION. AVOID BREATHING DUST. MAY BE HARMFUL IF INGESTED OR INHALED. WASH THOROUGHLY AFTER HANDLING. AVOID EXPOSURE TO LIGHT AND MOISTURE. PRESERVE IN WELL-CLOSED,LIGHT RESISTANT CONTAINER. STORE AT ROOM TEMPERATURE. -
INGREDIENTS AND APPEARANCE
CISAPRIDE MONOHYDRATE
cisapride monohydrate powderProduct Information Product Type Item Code (Source) NDC:51927-0257(NDC:66577-023) Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CISAPRIDE MONOHYDRATE (UNII: VZV0A4I38W) (CISAPRIDE - UNII:UVL329170W) CISAPRIDE MONOHYDRATE 1 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51927-0257-0 1 kg in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date bulk ingredient for animal drug compounding 07/15/2013 Labeler - Professional Compounding Centers of America dba PCCA (047919147) Establishment Name Address ID/FEI Business Operations Professional Compounding Centers of America dba PCCA 047919147 repack