URSOFERRAN- iron dextran solution
Ceva Sante Animale
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
URSOFERRAN® 200 mg/ml
Solution for injection for pigs
Name and address of the marketing authorisation holder and of the manufacturing authorisation holder responsible for batch release, if different
Marketing authorisation holder:
Serumwerk Bernburg AG
Hallesche Landstr. 105 b
06406 Bernburg, Germany
Manufacturer responsible for batch release:
Serumwerk Bernburg AG
Hallesche Landstr. 105 b
06406 Bernburg, Germany
Name of the veterinary medicinal product
URSOFERRAN 200 mg/ml
Solution for injection for pigs
Iron(III)-Ions (as Gleptoferron)
Statement of the active substance(s) and other ingredient(s)
Each ml contains:
Active substances:
Iron(III)-Ions 200.0 mg
(as Gleptoferron 532.6 mg)
Excipients:
Phenol 5.0 mg
A dark brown, slightly viscous, sterile, colloidal,
aqueous solution.
Contraindications
Do not administer to piglets suspected to suffer from deficiency of vitamin E and / or selenium. Do not use in cases of hypersensitivity to the active substance or to any of the excipients.
Do not use in clinically diseased animals, especially not in case of diarrhoea.
Adverse reactions
Uncommonly discolouration of the tissue and / or slight, soft swelling may be observed at the site of injection. This should disappear within a few days. Also hypersensitivity reactions can occur.
Rarely deaths have occurred in piglets following the administration of parenteral iron dextran preparations. These deaths have been associated with genetic factors or deficiency of vitamin E and/or selenium.
Very rarely piglets deaths have been reported which have been attributed to an increased susceptibility to infection due to temporary
blocking of the reticuloendothelial system.
The frequency of adverse reactions is defined using the following convention:
– very common (more than 1 in 10 animals treated displaying adverse reaction(s) during the course of one treatment)
– common (more than 1 but less than 10 animals in 100 animals treated)
– uncommon (more than 1 but less than 10 animals in 1,000 animals treated )
– rare (more than 1 but less than 10 animals in 10,000 animals treated)
– very rare (less than 1 animal in 10,000 animals treated, including isolated reports).
If you notice any side effects, even those not already listed in this package leaflet or you think that the medicine has not worked, please inform your veterinary surgeon.
Alternatively you can report via your national reporting system. For details regarding the national system please contact the national
competent authority.
Target species
Pig (piglet)
Dosage for each species, route(s) and method of administration
For strictly intramuscular injection.
Piglets:
200 mg Fe3+ per animal which is equivalent to 1 ml of the product per animal.
Inject once between the 1st and the 3rd day of life.
The use of a multidose syringe is recommended. To refill the syringe use a draw-off needle to avoid excessive broaching of the stopper. The stopper must not be broached more than 10 times. When treating groups of animals in one run, use a draw-off needle that has been placed in the vial stopper to avoid excess broaching of the stopper. The draw-off needle has to be removed after treatment.
Advice on correct administration
None
Special storage precautions
Keep out of the sight and reach of children.
Do not freeze.
Do not use after the expiry date stated on the label.
Shelf-life after first opening the container: 28 days.
When the container is broached (opened) for the first time, using the in-use shelf-life which is specified on this package insert, the date on
which any product remaining in the container should be discarded should be worked out.
This discard date should be written in the space provided on the label.
Special warnings
Special precautions to be taken by the person administering the veterinary medicinal product to animals:
People with known hypersensitivity to iron dextran, or those with haemochromatosis should avoid contact with the product.
Care should be taken to avoid accidental self injection, as well as contact with the eyes and mouth.
In case of accidental injection, seek medical advice immediately and show the package leaflet or label to the physician.
Wash hands after use.
Use during pregnancy or lactation:
Not applicable
Interaction with other medicinal products and other forms of interaction:
The absorption of concomitantly administered oral iron may be reduced. See also under section “Incompatibilities”.
Overdose (symptoms, emergency procedures, antidotes), if necessary:
Transferrin-iron saturation levels leading to increased susceptibility for (systemic) bacterial disease, pain, inflammation reactions as well as
abscess formation at the injection site may occur.
Persistent discolouration of muscle tissue at the injection site may occur.
Iatrogenic poisoning with following symptoms: pale mucous membranes, hemorrhagic gastroenteritis, vomiting, tachycardia, hypotension, dyspnoea, edema of the limbs, lameness, shock, death, liver damage. Supportive measures such as chelating agents can be used.
Incompatibilities:
In the absence of compatibility studies, this veterinary medicinal product must not be mixed with other veterinary medicinal products.
Special precautions for the disposal of unused product or waste materials, if any
Unused veterinary medicinal products should preferably be returned to special waste collection points. When disposed of together with household waste, it must be ensured that this waste cannot be misused. Veterinary medicinal products must not be disposed of via wastewater or sewerage.
Date on which the package leaflet was last approved
December 2017
Other information
Nature and composition of immediate packaging:
100 ml clear glass vial (type II) with chlorobutyl rubber closure (type I) and aluminium / polypropylene cap
Carton box with 1 glass vial with 100 ml
Client Information about
URSOFERRAN® 200 mg/ml
Solution for injection for pigs
The Client Information Sheet contains important information about URSOFERRAN. This sheet of information is provided only as supplemental information to the package insert. For more detailed information about URSOFERRAN, refer to the product package insert.
URSOFERRAN is a solution for injection containing iron(III) ions as gleptoferron, a macro molecular complex containing iron in a readily utilizable form for the prevention and treatment of iron deficiency anaemia in piglets. The solution contains phenol as a preservative.
URSOFERRAN is not an approved drug product in the U.S. URSOFERRAN is authorized in Germany. Due to a potential animal drug shortage, the U.S. Food and Drug Administration is temporarily permitting marketing of UROSFERRAN in the U.S. for prevention or treatment of anemia in baby pigs.
To report a suspected adverse reaction, complaint or product quality defect, call Ceva’s Product Support Team at 1-800-999-0297. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
MARKETED BY:
Ceva Biomune, Lenexa, KS 66215, 1-800-999-0297
URSOFERRAN® is a registered trademark of Serumwerk Bernburg AG.
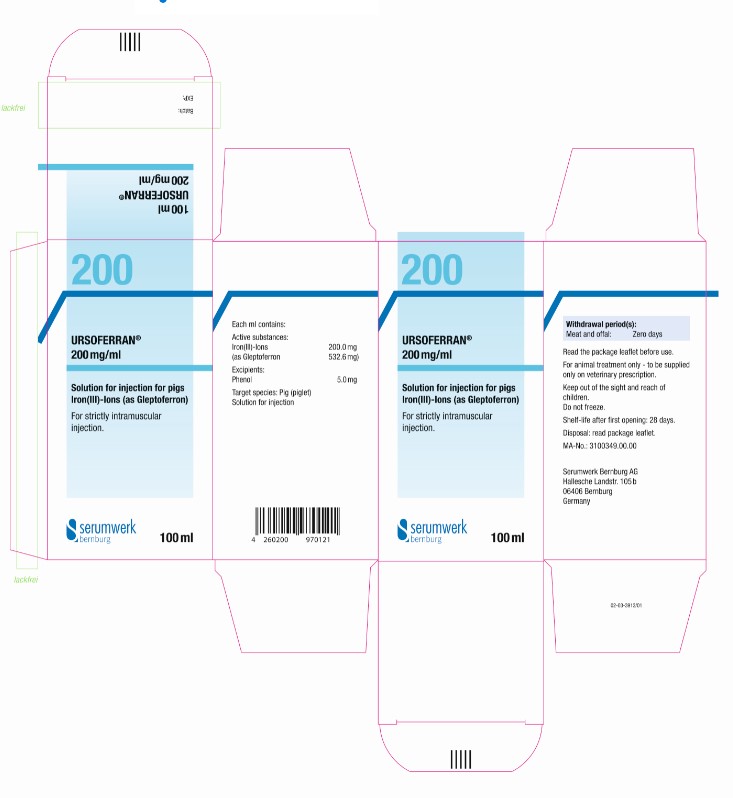
PRINCIPAL DISPLAY PANEL - 100 mL Bottle Label
URSOFERRAN® 200 mg/ml
100ml
Solution for injection for pigs
Iron(III)-Ions (as Gleptoferron)
Active substances: Iron(III)-Ions 200.0 mg
(as Gleptoferron 532.6 mg)
Excipients: Phenol 5.0 mg
Solution for injection
For strictly intramuscular injection.
Read the package leaflet before use.
Target Species: Pig (piglet)
Withdrawal period(s): Meat and offal: Zero days
Shelf-life after first opening: 28 days.
Once broached, use by ......................................
Do not freeze.
For animal treatment only - to be supplied only on veterinary prescription.
Keep out of sight and reach of children.

| URSOFERRAN
iron dextran solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ceva Sante Animale (261126049) |
| Registrant - Ceva Sante Animale (261126049) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Serumwerk Bernburg AG | 330105057 | api manufacture, manufacture | |