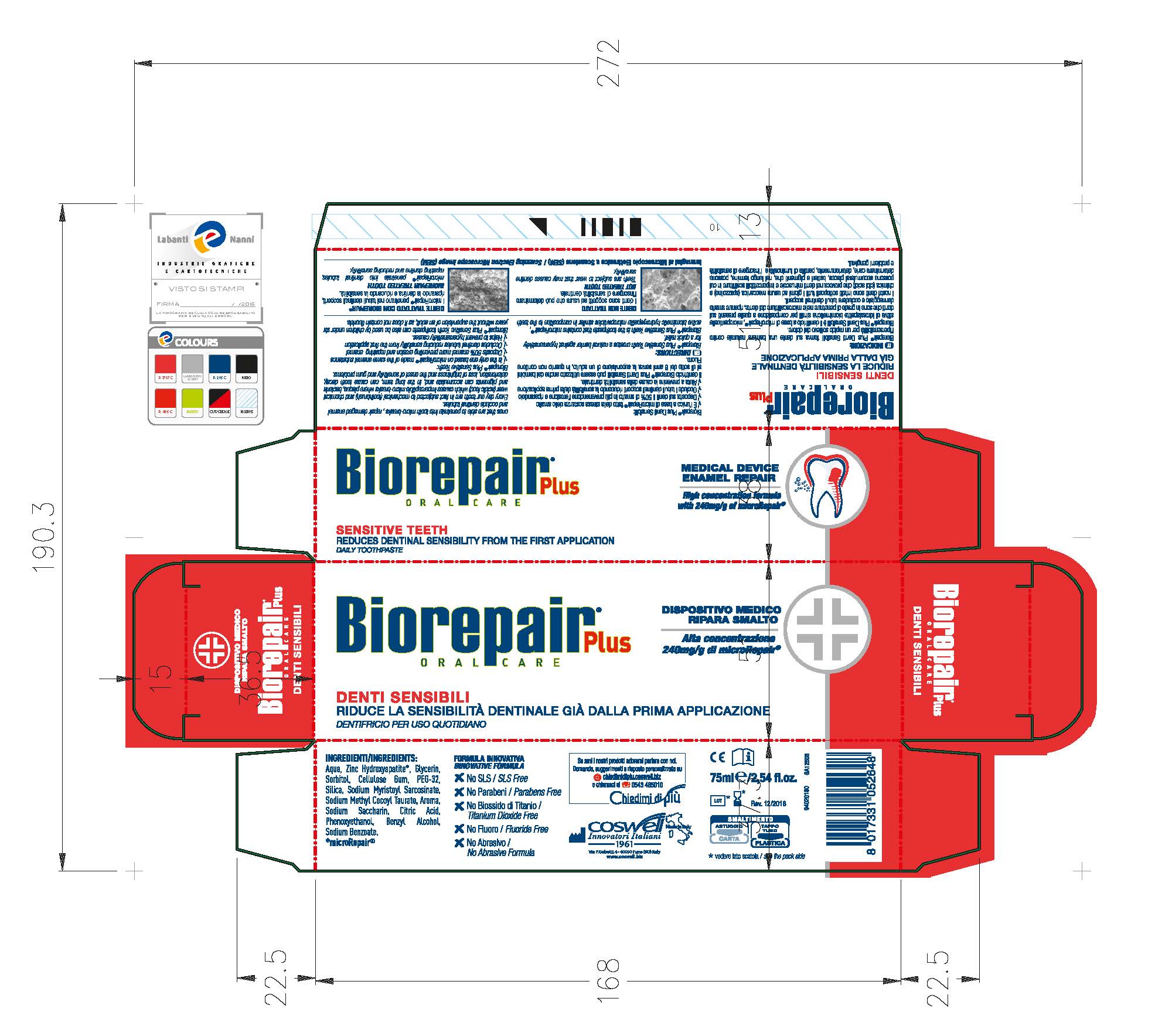
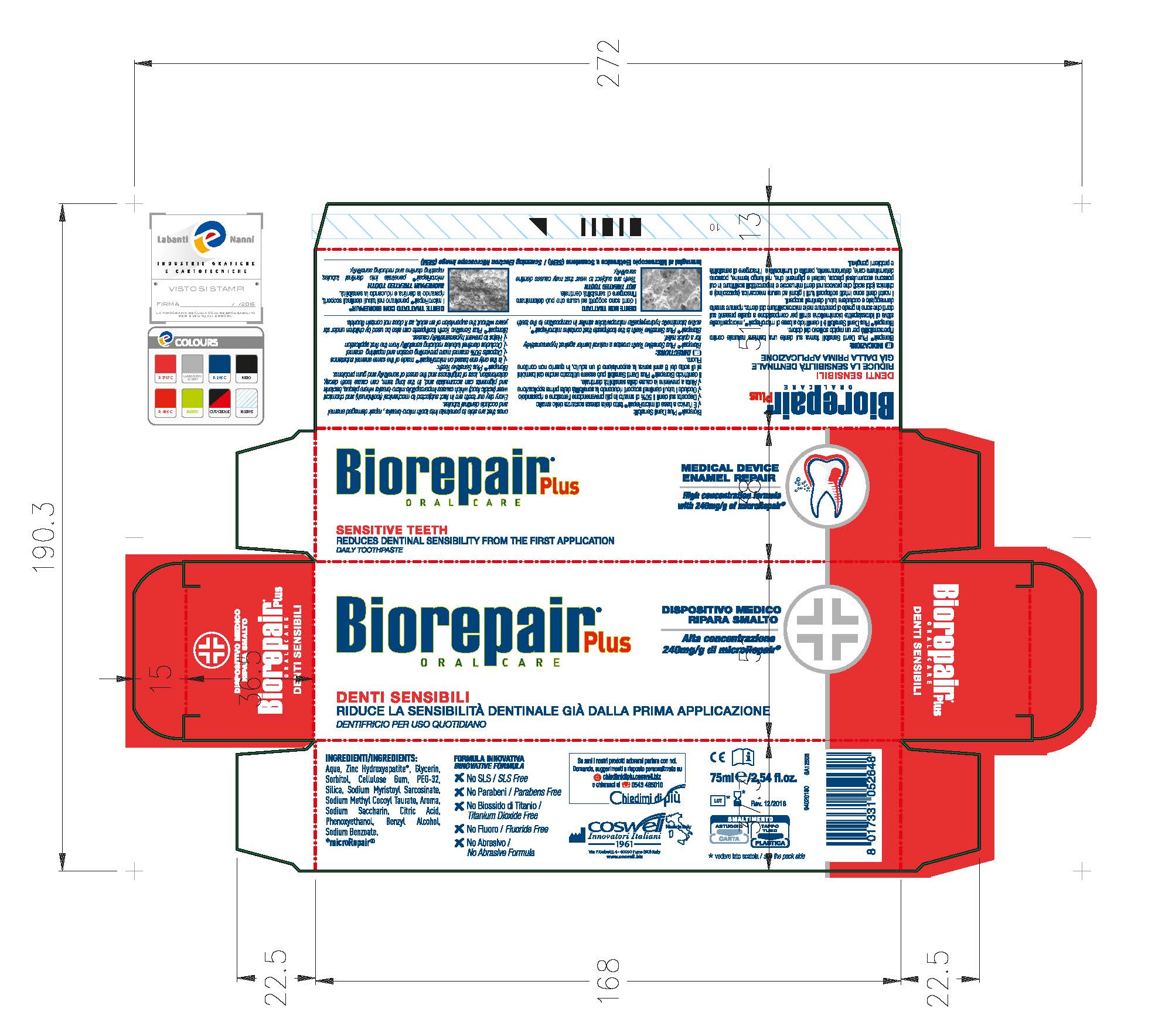
Label: BIOREPAIR SENSITIVE TEETH- dicalcium phosphate dihydrate paste
-
Contains inactivated NDC Code(s)
NDC Code(s): 70781-006-01 - Packager: Coswell Spa
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 13, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
-
PURPOSE
Biorepair Plus Sensitive Teeth creates a natural barrier against hypersensitivity for a quick relief.
Biorepair Plus Sensitive Teeth is the toothpaste that contains microRepair, active biomimetic hydroxyapatite microparticles similar in composition to the teeth ones that are able to penetrate into micro-breaks, repair damaged enamel and occlude dentinal tubules.
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIOREPAIR SENSITIVE TEETH
dicalcium phosphate dihydrate pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70781-006 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) (ANHYDROUS DIBASIC CALCIUM PHOSPHATE - UNII:L11K75P92J) ANHYDROUS DIBASIC CALCIUM PHOSPHATE 18.6 g in 100 g Inactive Ingredients Ingredient Name Strength HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM MYRISTOYL SARCOSINATE (UNII: J07237209D) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) BENZYL ALCOHOL (UNII: LKG8494WBH) PHENOXYETHANOL (UNII: HIE492ZZ3T) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70781-006-01 75 g in 1 TUBE; Type 0: Not a Combination Product 06/02/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/02/2017 Labeler - Coswell Spa (429512304) Establishment Name Address ID/FEI Business Operations Incos Cosmeceutica Industriale Srl 434933032 manufacture(70781-006)