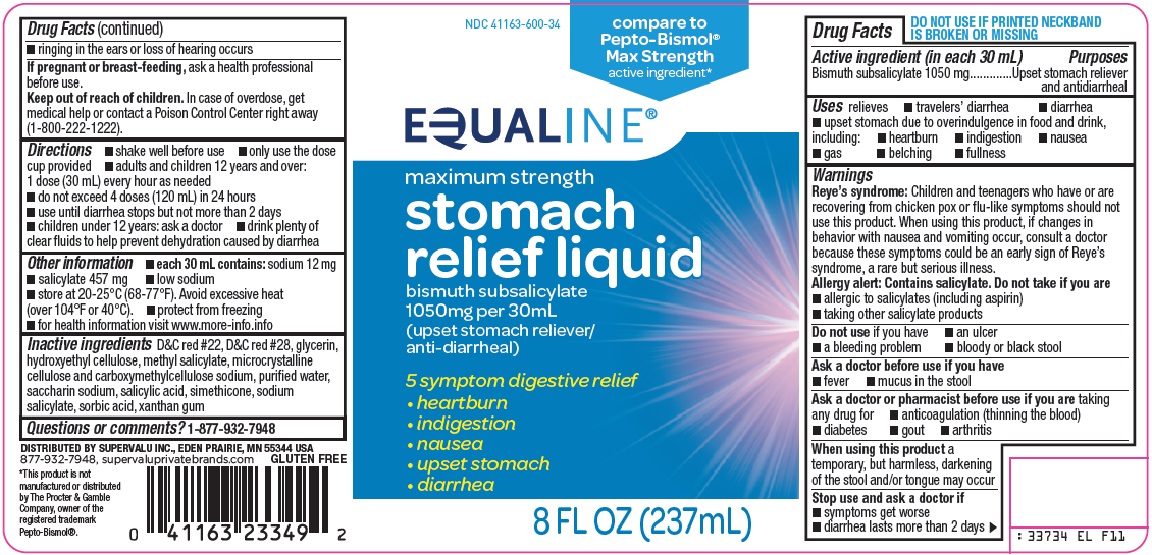
EQUALINE STOMACH RELIEF- bismuth subsalicylate suspension
Supervalu Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SuperValu Inc. Stomach Relief Liquid Drug Facts
Uses
relieves
- •
- travelers’ diarrhea
- •
- diarrhea
- •
- upset stomach due to overindulgence in food and drink, including:
- •
- heartburn
- •
- indigestion
- •
- nausea
- •
- gas
- •
- belching
- •
- fullness
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- •
- allergic to salicylates (including aspirin)
- •
- taking other salicylate products
Ask a doctor or pharmacist before use if you are
taking any drug for
- •
- anticoagulation (thinning the blood)
- •
- diabetes
- •
- gout
- •
- arthritis
Directions
- •
- shake well before use
- •
- only use the dose cup provided
- •
- adults and children 12 years and over: 1 dose (30 mL) every hour as needed
- •
- do not exceed 4 doses (120 mL) in 24 hours
- •
- use until diarrhea stops but not more than 2 days
- •
- children under 12 years: ask a doctor
- •
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
Other information
- •
- each 30 mL contains: sodium 12 mg
- •
- salicylate 457 mg
- •
- low sodium
- •
- store at 20-25°C (68-77°F). Avoid excessive heat (over 104°F or 40°C).
- •
- protect from freezing
- •
- for health information visit www.more-info.info
| EQUALINE STOMACH RELIEF
bismuth subsalicylate suspension |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Supervalu Inc (006961411) |
Revised: 4/2020
Document Id: a5b65fe7-137a-41f5-beac-114cb879a3cd
Set id: 50f99895-8fd0-48c2-be5e-8fdb9c7e506d
Version: 6
Effective Time: 20200415
Supervalu Inc