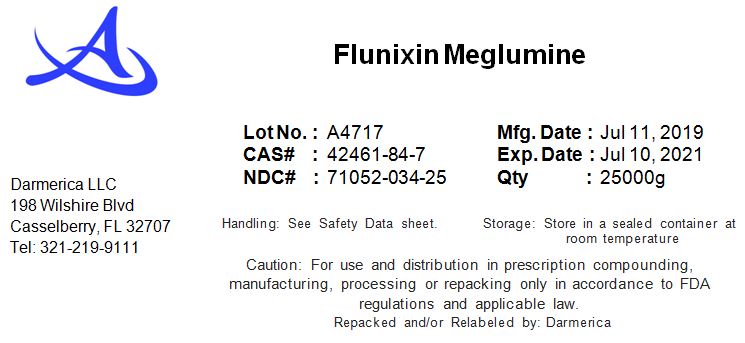
FLUNIXIN MEGLUMINE- flunixin meglumine powder
Darmerica, LLC
----------
Flunixin Meglumine
| FLUNIXIN MEGLUMINE
flunixin meglumine powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Darmerica, LLC (080233052) |
Revised: 1/2021
Document Id: b98070dd-8e5a-7eef-e053-2a95a90ac65c
Set id: 504933ad-e3ee-71f0-e054-00144ff88e88
Version: 6
Effective Time: 20210122
Darmerica, LLC