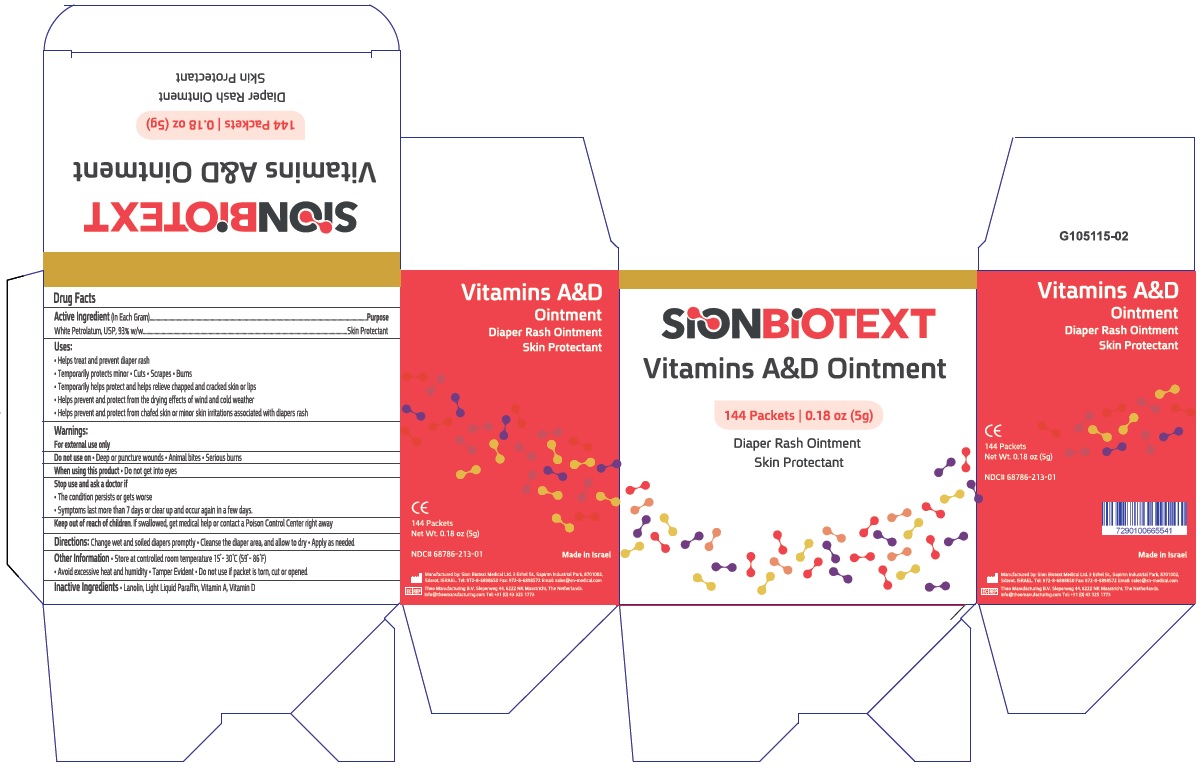
Label: VITAMIN A D- white petrolatum ointment
-
NDC Code(s):
68786-213-01,
68786-213-02,
68786-213-03,
68786-213-04, view more68786-213-05
- Packager: Sion Biotext Medical Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
Uses
- Helps treat and prevent diaper rash
- Helps seal out wetness
- Temporarily protects minor * cuts * scrapes * burns
- Temporarily helps protect and help relieve chapped and cracked skin and lips
- Helps prevent and protect from the drying effects of wind and cold weather
- Helps prevent and protect chafed skin or minor skin irritations associated with diaper rash
- With each diaper change, especially at bedtime when exposure to wet diapers may be prolonged
- Warnings:
- Directions
- Other information:
- Inactive Ingredients
-
Principal Display panel
NDC 68786-213-01
Vitamins A&D Ointment
Intensive Moisturizer for Skin Therapy
For Personal and Professional Use
SION MEDICAL
Made in Israel
144 Packets
Net Wt. 0.17oz(5g)

NDC 68786-213-02
Vitamins A&D Ointment
Intensive Moistuirzer for Skin Therapy
For Personal and Professional Use
SION MEDICAL
Made in Israel
4 tubes
Net Wt 4 oz (113 g)

NDC 68786-213-03
Vitamins A&D Ointment
Diaper Rash Ointment
Skin Protectant
Made in Israel
12 tubes
Net Wt 4 oz (113 g)

NDC 68786-213-04
Vitamins A&D Ointment
Diaper Rash Ointment
Skin Protectant
Made in Israel
1 tube
Net Wt 4 oz (113 g)

NDC 68786-213-05
Vitamins A&D Ointment
Diaper Rash Ointment
Skin Protectant
Made in Israel
144 Packets
0.18 oz (5 g)
-
INGREDIENTS AND APPEARANCE
VITAMIN A D
white petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68786-213 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 0.93 g in 1 g Inactive Ingredients Ingredient Name Strength VITAMIN A (UNII: 81G40H8B0T) VITAMIN D (UNII: 9VU1KI44GP) LANOLIN (UNII: 7EV65EAW6H) LIGHT MINERAL OIL (UNII: N6K5787QVP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68786-213-01 6 in 1 CASE 02/14/2017 1 144 in 1 BOX 1 5 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC:68786-213-02 18 in 1 CASE 02/14/2017 2 4 in 1 BOX 2 113 g in 1 PACKET; Type 0: Not a Combination Product 3 NDC:68786-213-03 6 in 1 CASE 01/31/2019 3 12 in 1 BOX 3 113 g in 1 PACKET; Type 0: Not a Combination Product 4 NDC:68786-213-04 56 in 1 CASE 02/14/2017 4 1 in 1 BOX 4 113 g in 1 TUBE; Type 0: Not a Combination Product 5 NDC:68786-213-05 6 in 1 CASE 02/14/2017 5 144 in 1 BOX 5 5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/14/2017 Labeler - Sion Biotext Medical Ltd. (532775194) Registrant - Sion Biotext Medical Ltd. (532775194)