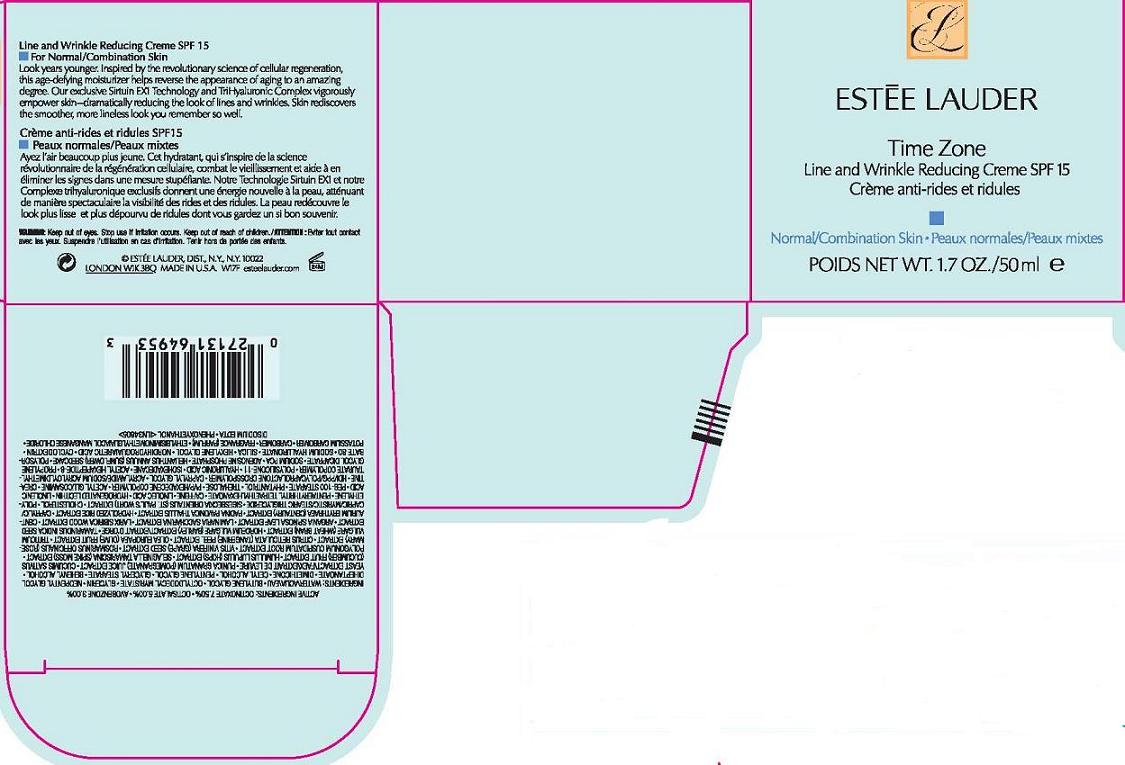
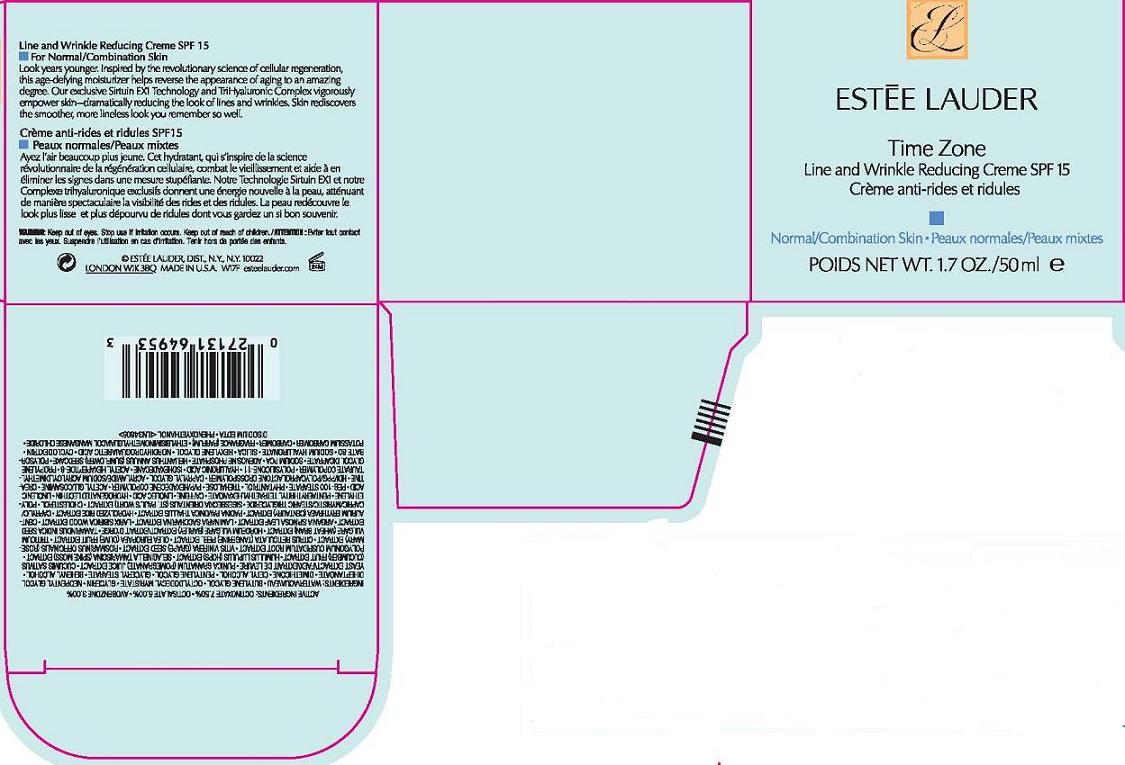
Label: TIME ZONE LINE AND WRINKLE REDUCING CREME SPF 15 NORMAL COMBO SKIN- octinoxate, octsalate, avobenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 11559-737-01, 11559-737-02 - Packager: ESTEE LAUDER INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 22, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS: WATER [] BUTYLENE GLYCOL [] OCTYLDODECYL MYRISTATE [] GLYCERIN [] NEOPENTYL GLYCOL DIHEPTANOATE [] DIMETHICONE [] CETYL ALCOHOL [] PENTYLENE GLYCOL [] GLYCERYL STEARATE [] BEHENYL ALCOHOL [] YEAST EXTRACT\FAEX\EXTRAIT DE LEVURE [] PUNICA GRANATUM (POMEGRANATE) JUICE EXTRACT [] CUCUMIS SATIVUS (CUCUMBER) FRUIT EXTRACT [] HUMULUS LUPULUS (HOPS) EXTRACT [] SELAGINELLA TAMARISCINA (SPIKE MOSS) EXTRACT [] POLYGONUM CUSPIDATUM ROOT EXTRACT [] VITIS VINIFERA (GRAPE) SEED EXTRACT [] ROSMARINUS OFFICINALIS (ROSEMARY) EXTRACT [] CITRUS RETICULATA (TANGERINE) PEEL EXTRACT [] OLEA EUROPAEA (OLIVE) FRUIT EXTRACT [] TRITICUM VULGARE (WHEAT BRAN) EXTRACT [] HORDEUM VULGARE (BARLEY) EXTRACT\EXTRAIT D'ORGE [] TAMARINDUS INDICA SEED EXTRACT [] ARGANIA SPINOSA LEAF EXTRACT [] LAMINARIA SACCHARINA EXTRACT [] LARIX SIBIRICA WOOD EXTRACT [] CENTAURIUM ERYTHRAEA (CENTAURY) EXTRACT [] PADINA PAVONICA THALLUS EXTRACT [] HYDROLYZED RICE EXTRACT [] CAPRYLIC/CAPRIC/MYRISTIC/STEARIC TRIGLYCERIDE [] SIGESBECKIA ORIENTALIS EXTRACT [] CHOLESTEROL [] POLYETHYLENE [] PENTAERYTHRITYL TETRAETHYLHEXANOATE [] CAFFEINE [] LINOLEIC ACID [] HYDROGENATED LECITHIN [] LINOLENIC ACID [] PEG-100 STEARATE [] PHYTANTRIOL [] TREHALOSE [] PVP/HEXADECENE COPOLYMER [] ACETYL GLUCOSAMINE [] CREATINE [] HDI/PPG/POLYCAPROLACTONE CROSSPOLYMER [] CAPRYLYL GLYCOL [] ACRYLAMIDE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER [] POLYSILICONE-11 [] HYALURONIC ACID [] ISOHEXADECANE [] ACETYL HEXAPEPTIDE-8 [] PROPYLENE GLYCOL DICAPRATE [] SODIUM PCA [] ADENOSINE PHOSPHATE [] HELIANTHUS ANNUUS (SUNFLOWER) SEEDCAKE [] POLYSORBATE 80 [] SODIUM HYALURONATE [] SILICA [] HEXYLENE GLYCOL [] NORDIHYDROGUAIARETIC ACID [] CYCLODEXTRIN [] POTASSIUM CARBOMER [] CARBOMER [] FRAGRANCE (PARFUM) [] ETHYLBISIMINOMETHYLGUAIACOL MANGANESE CHLORIDE [] DISODIUM EDTA [] PHENOXYETHANOL
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TIME ZONE LINE AND WRINKLE REDUCING CREME SPF 15 NORMAL COMBO SKIN
octinoxate, octsalate, avobenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11559-737 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mL in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5.0 mL in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3.0 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) CUCUMBER (UNII: YY7C30VXJT) OLIVE OIL (UNII: 6UYK2W1W1E) BARLEY (UNII: 5PWM7YLI7R) TAMARIND SEED (UNII: 6AHP8A7OML) SACCHARINA LATISSIMA WHOLE (UNII: RTR3OGP8ET) CHOLESTEROL (UNII: 97C5T2UQ7J) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) LINOLEIC ACID (UNII: 9KJL21T0QJ) TREHALOSE (UNII: B8WCK70T7I) CREATINE (UNII: MU72812GK0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POLYSORBATE 60 (UNII: CAL22UVI4M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) EDETATE DISODIUM (UNII: 7FLD91C86K) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11559-737-01 1 in 1 CARTON 1 NDC:11559-737-02 50 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2009 Labeler - ESTEE LAUDER INC (005914387) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 205952385 manufacture Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 manufacture Establishment Name Address ID/FEI Business Operations Len-Ron Manufacturing Division of Aramis Inc. 809771152 manufacture Establishment Name Address ID/FEI Business Operations Aramis Inc. 042918826 manufacture Establishment Name Address ID/FEI Business Operations Northtec Bristol 949264774 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Northtec Keystone 618107429 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Pennsylvania Distribution Center 2 828534516 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd. 255175580 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd 253616536 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Distribution Center 208579636 repack, relabel Establishment Name Address ID/FEI Business Operations Estee Lauder Kabushiki Kaisha 712808195 relabel, repack Establishment Name Address ID/FEI Business Operations Whitman Laboratories Ltd. 216866277 manufacture Establishment Name Address ID/FEI Business Operations Aveda Corporation 071352058 manufacture