DERMAKLEAR AKNE TREATMENT WITH SULFUR- sulfur soap
Schwabe North America, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
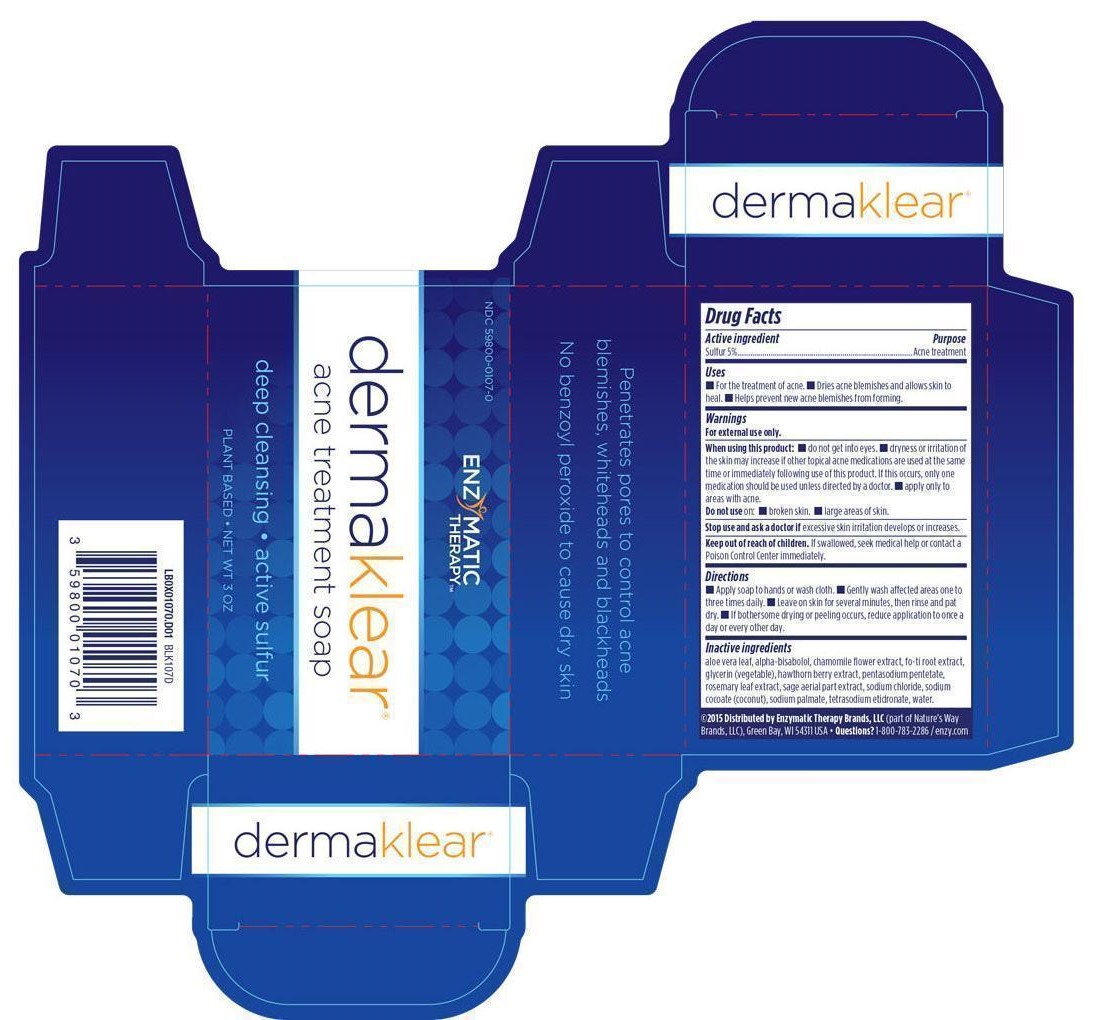
DermaKlear Akne Treatment with Sulfur
Inactive Ingredient
Aloe vera leaf
alpha-bisabolol
Chamomile flower extract
fo-ti root extract
glycerin (vegetable)
Hawthorn berry extract
Pentasodium pentetate
Rosemary leaf extract
Sage aerial part extract
Sodium chloride
Sodium cocoate (coconut)
Sodium palmate
Tetrasodium etidronate
Water
Dosgae & Administration
Directions: Apply soap to hands or wash cloth.
Gently wash affected areas one to three times daily.
Leave on skin for several minutes, then rinse and pat dry.
If bothersome drying or peeling occurs, reduce application to once a day or every other day.
Indications & Usage
For the treatment of acne.
Dries acne blemishes and allows skin to heal.
Helps prevent new acne blemishes from forming.
Purpose
For the treatment of acne.
Dries acne blemishes and allows skin to heal.
Helps prevent new acne blemishes from forming.
When using
When using this product do not get into eyes; dryness or irritation of the skin may increase if other topical acne medications are used at the same time or immediately following use of this product.
If this occurs only one medication should be used unless directed by a doctor.
Apply only to areas with acne.
Do not use
Do Not Use on broken skin or large areas of skin.
Stop use and ask a doctor if excessive skin irritation develops or increases.
| DERMAKLEAR AKNE TREATMENT WITH SULFUR
sulfur soap |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Schwabe North America, Inc (831153908) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bradford Soap Works, Inc | 001201045 | manufacture(59800-0107) | |