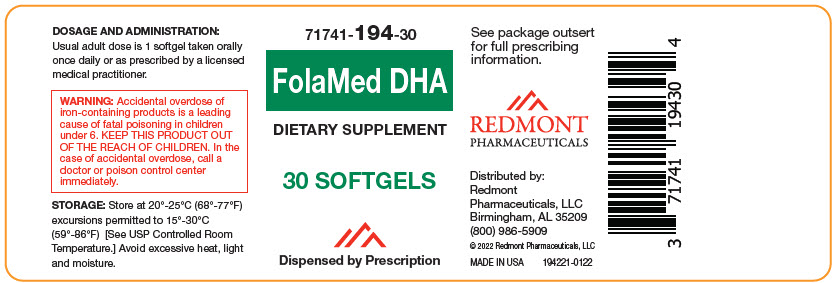
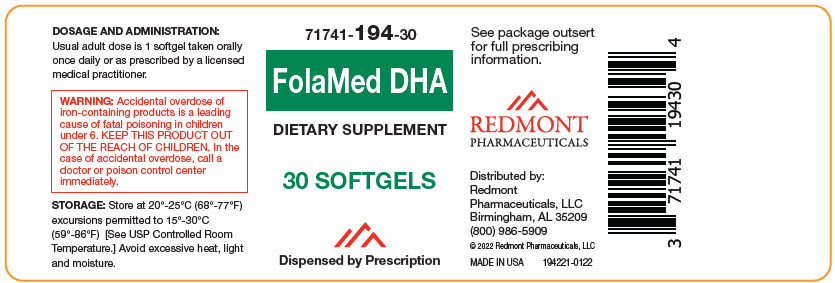
Label: FOLAMED DHA- beta carotene, vitamin a palmitate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, d-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, calcium pantothenate, calcium carbonate, iron, potassium iodide, magnesium oxide, zinc oxide, cupric oxide, and fish oil capsule, gelatin coated
- NHRIC Code(s): 71741-194-30
- Packager: Redmont Pharmaceuticals, LLC
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated January 27, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HEALTH CLAIM
71741-194-30
Multivitamin
Dietary Supplement
Dispensed by Prescription1
- 1
- This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking B12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (64 FR 8760). 1-3 The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription. This is not an Orange Book product. This product may be administered only under a physician's supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size: 1 Softgel Serving Per Container: Amount Per Serving % Daily Value Other Ingredients: Bovine Gelatin, Glycerin, Yellow Beeswax, Purified Water, Soy Lecithin, Annatto. - *
- Daily Values not established
Calories 5 Total Fat 0.5 g <1% Cholesterol 25 mg 8% Vitamin A (50% Beta-Carotene, 50% Retinyl Palmitate) 1200 mcg RAE (4000 IU) 133% Vitamin C (as Ascorbic Acid) 60 mg 67% Vitamin D (as Cholecalciferol) 10 mcg 50% Vitamin E (as d-Alpha Tocopherol) 20.1 mg (30 IU) 134% Thiamin (B1) (from Thiamine Mononitrate) 1.7 mg 142% Riboflavin (B2) 2 mg 154% Niacin (as Niacinamide) 20 mg 125% Vitamin B6 (from Pyridoxine Hydrochloride) 2.5 mg 147% Folate (1000 mcg Folic Acid) 1700 mcg DFE 425% Vitamin B12 (as Cyanocobalamin) 8 mcg 333% Biotin 300 mcg 1000% Pantothenic Acid (from d-Calcium Pantothenate) 10 mg 200% Calcium (from Calcium Carbonate) 200 mg 15% Iron (from Carbonyl Iron) 28 mg 156% Iodine (from Potassium Iodide) 150 mcg 100% Magnesium (from Magnesium Oxide) 50 mg 12% Zinc (from Zinc Oxide) 15 mg 136% Cooper (Cooper Oxide) 2 mg 222% Eicosapentaenoic Acid (as EE) (from Fish Oil) 200 mg * Docosahexaenoic Acid (as EE) (from Fish Oil) 35 mg * Allergens: Fish (Anchovy, Sardine, Tuna), Soy.
Does Not Contain: Peanuts, Tree Nuts, Shellfish, Eggs, Milk, Wheat.
-
INDICATIONS
FolaMed DHA is prescription dietary supplement formulated for the clinical dietary management of suboptimal nutritional status in patients where advanced folate supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health.
FolaMed DHA is formulated as a prescription dietary supplement for use in the dietary management of patients with nutritional deficiencies or are in need of nutritional supplementation.
- CONTRAINDICATIONS
- PRECAUTIONS
-
WARNINGS
WARNING: Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding. WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. -
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
You should call your doctor for medical advice about serious adverse events. To report adverse side effects or to obtain product information, contact Redmont Pharmaceuticals, LLC at 1-800-986-5909.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Child-resistant bottles of 30 softgels (71741-194-302). Softgel is oblong shaped and orange reddish in color with possible dark spots.
- Federal Register Notice of August 2, 1973 (38 FR 20750)
- Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
- Federal Register Notice of March 5, 1996 (61 FR 8760)
- 2
- Redmont Pharmaceuticals does not represent this product code to be National Drug Code (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
- STORAGE AND HANDLING
-
HEALTH CLAIM
Distributed by:
Redmont Pharmaceuticals, LLC
Birmingham, AL 35209
800-986-5909These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
© 2022 Redmont Pharmaceuticals, LLC
194111-0122 - PRINCIPAL DISPLAY PANEL - 30 Softgel Bottle Label
-
INGREDIENTS AND APPEARANCE
FOLAMED DHA
beta carotene, vitamin a palmitate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, d-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, calcium pantothenate, calcium carbonate, iron, potassium iodide, magnesium oxide, zinc oxide, cupric oxide, and fish oil capsule, gelatin coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:71741-194 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETA CAROTENE (UNII: 01YAE03M7J) (BETA CAROTENE - UNII:01YAE03M7J) BETA CAROTENE 600 ug Vitamin A Palmitate (UNII: 1D1K0N0VVC) (Retinol - UNII:G2SH0XKK91) Retinol 600 ug Ascorbic Acid (UNII: PQ6CK8PD0R) (Ascorbic Acid - UNII:PQ6CK8PD0R) Ascorbic Acid 60 mg Cholecalciferol (UNII: 1C6V77QF41) (Cholecalciferol - UNII:1C6V77QF41) Cholecalciferol 10 ug .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 20.1 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.7 mg Riboflavin (UNII: TLM2976OFR) (Riboflavin - UNII:TLM2976OFR) Riboflavin 2 mg Niacinamide (UNII: 25X51I8RD4) (Niacinamide - UNII:25X51I8RD4) Niacinamide 20 mg Pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) Pyridoxine Hydrochloride 2.5 mg Folic Acid (UNII: 935E97BOY8) (Folic Acid - UNII:935E97BOY8) Folic Acid 1000 ug Cyanocobalamin (UNII: P6YC3EG204) (Cyanocobalamin - UNII:P6YC3EG204) Cyanocobalamin 8 ug Biotin (UNII: 6SO6U10H04) (Biotin - UNII:6SO6U10H04) Biotin 300 ug Calcium Pantothenate (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 10 mg Calcium Carbonate (UNII: H0G9379FGK) (Calcium Cation - UNII:2M83C4R6ZB) Calcium Carbonate 200 mg Iron (UNII: E1UOL152H7) (Iron - UNII:E1UOL152H7) Iron 28 mg Potassium Iodide (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) Potassium Iodide 150 ug Magnesium Oxide (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) Magnesium Oxide 50 mg Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 15 mg Cupric Oxide (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg Fish Oil (UNII: XGF7L72M0F) (Fish Oil - UNII:XGF7L72M0F) Fish Oil 235 mg Inactive Ingredients Ingredient Name Strength Gelatin Type B Bovine (150 Bloom) (UNII: F5AJW0ONK4) Glycerin (UNII: PDC6A3C0OX) Yellow Wax (UNII: 2ZA36H0S2V) Water (UNII: 059QF0KO0R) Lecithin, Soybean (UNII: 1DI56QDM62) Annatto (UNII: 6PQP1V1B6O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71741-194-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 01/13/2022 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 20 mm Labeler - Redmont Pharmaceuticals, LLC (080843607)