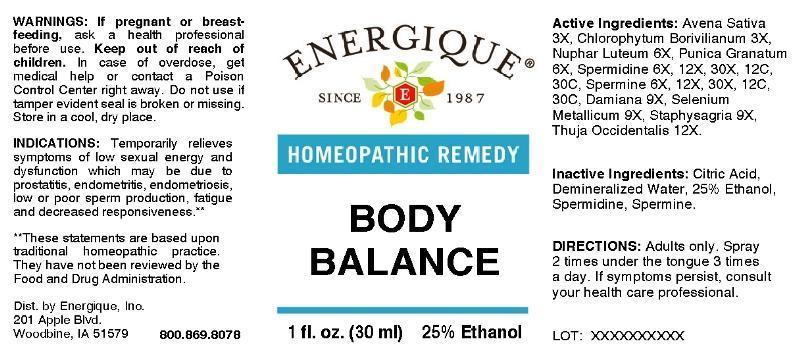
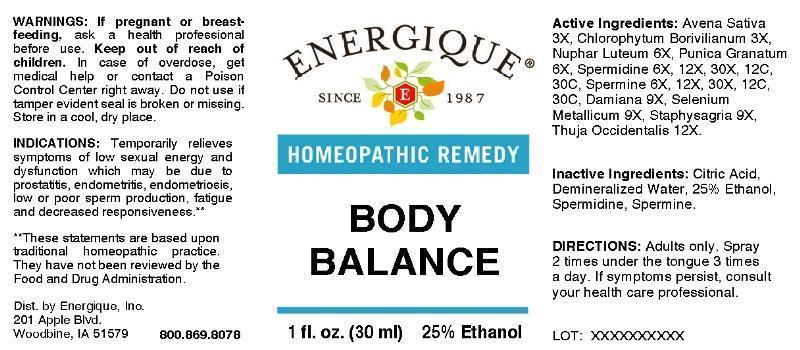
Label: BODY BALANCE- avena sativa, chlorophytum borivilianum, nuphar luteum, punica granatum, spermidine, spermine, damiana, selenium metallicum, staphysagria, thuja occidentalis liquid
- NDC Code(s): 44911-0110-1
- Packager: Energique, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 14, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS:
-
INDICATIONS:
Temporarily relieves symptoms of low sexual energy and dysfunction which may be due to prostatitis, endometritis, endometriosis, low or poor sperm production, fatigue and decreased responsiveness.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
-
INDICATIONS:
Temporarily relieves symptoms of low sexual energy and dysfunction which may be due to prostatitis, endometritis, endometriosis, low or poor sperm production, fatigue and decreased responsiveness.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE DISPLAY LABEL:
-
INGREDIENTS AND APPEARANCE
BODY BALANCE
avena sativa, chlorophytum borivilianum, nuphar luteum, punica granatum, spermidine, spermine, damiana, selenium metallicum, staphysagria, thuja occidentalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44911-0110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) (AVENA SATIVA FLOWERING TOP - UNII:MA9CQJ3F7F) AVENA SATIVA FLOWERING TOP 3 [hp_X] in 1 mL ASPARAGUS ADSCENDENS ROOT (UNII: PCV2634GQH) (ASPARAGUS ADSCENDENS ROOT - UNII:PCV2634GQH) ASPARAGUS ADSCENDENS ROOT 3 [hp_X] in 1 mL NUPHAR LUTEUM ROOT (UNII: 714LIU3V6D) (NUPHAR LUTEUM ROOT - UNII:714LIU3V6D) NUPHAR LUTEUM ROOT 6 [hp_X] in 1 mL POMEGRANATE (UNII: 56687D1Z4D) (POMEGRANATE - UNII:56687D1Z4D) POMEGRANATE 6 [hp_X] in 1 mL SPERMIDINE (UNII: U87FK77H25) (SPERMIDINE - UNII:U87FK77H25) SPERMIDINE 6 [hp_X] in 1 mL SPERMINE (UNII: 2FZ7Y3VOQX) (SPERMINE - UNII:2FZ7Y3VOQX) SPERMINE 6 [hp_X] in 1 mL TURNERA DIFFUSA LEAFY TWIG (UNII: RQ2CFA7WWJ) (TURNERA DIFFUSA LEAFY TWIG - UNII:RQ2CFA7WWJ) TURNERA DIFFUSA LEAFY TWIG 9 [hp_X] in 1 mL SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 9 [hp_X] in 1 mL DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 9 [hp_X] in 1 mL THUJA OCCIDENTALIS LEAF (UNII: 0T0DQN8786) (THUJA OCCIDENTALIS LEAF - UNII:0T0DQN8786) THUJA OCCIDENTALIS LEAF 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44911-0110-1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 12/02/2013 08/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/02/2013 08/26/2024 Labeler - Energique, Inc. (789886132) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(44911-0110) , api manufacture(44911-0110) , label(44911-0110) , pack(44911-0110)