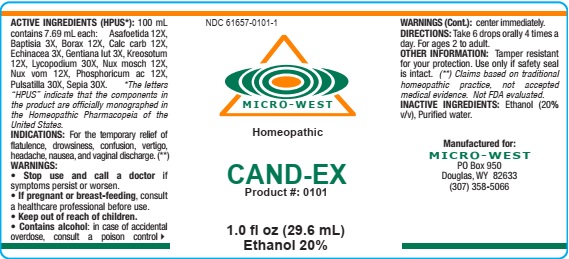
Label: CAND-EX- lycopodium,nux vomica,borax,asafoetida,nux moschata,gentiana lutea,kreosotum,calcarea carbonica,sepia,phosphoric acid,echinacea,pulsatilla,baptisia, liquid
- NDC Code(s): 61657-0101-1
- Packager: WHITE MANUFACTURING INC. DBA MICRO WEST
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 21, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- Inactive ingredients
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS
- WARNING
- DIRECTIONS
- Other Information
- MANUFACTURE
- LABEL
-
INGREDIENTS AND APPEARANCE
CAND-EX
lycopodium,nux vomica,borax,asafoetida,nux moschata,gentiana lutea,kreosotum,calcarea carbonica,sepia,phosphoric acid,echinacea,pulsatilla,baptisia, liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61657-0101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_X] in 30 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 12 [hp_X] in 30 mL ECHINACEA, UNSPECIFIED (UNII: 4N9P6CC1DX) (ECHINACEA, UNSPECIFIED - UNII:4N9P6CC1DX) ECHINACEA, UNSPECIFIED 3 [hp_X] in 30 mL BAPTISIA TINCTORIA (UNII: 5K1UO2888Y) (BAPTISIA TINCTORIA - UNII:5K1UO2888Y) BAPTISIA TINCTORIA 3 [hp_X] in 30 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_X] in 30 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_X] in 30 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 12 [hp_X] in 30 mL WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 12 [hp_X] in 30 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 12 [hp_X] in 30 mL SODIUM BORATE (UNII: 91MBZ8H3QO) (BORATE ION - UNII:44OAE30D22) SODIUM BORATE 12 [hp_X] in 30 mL GENTIANA LUTEA ROOT (UNII: S72O3284MS) (GENTIANA LUTEA ROOT - UNII:S72O3284MS) GENTIANA LUTEA ROOT 3 [hp_X] in 30 mL NUTMEG (UNII: AEE24M3MQ9) (NUTMEG - UNII:AEE24M3MQ9) NUTMEG 12 [hp_X] in 30 mL ASAFETIDA (UNII: W9FZA51AS1) (ASAFETIDA - UNII:W9FZA51AS1) ASAFETIDA 12 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61657-0101-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 08/01/1993 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/01/1993 Labeler - WHITE MANUFACTURING INC. DBA MICRO WEST (082307831)