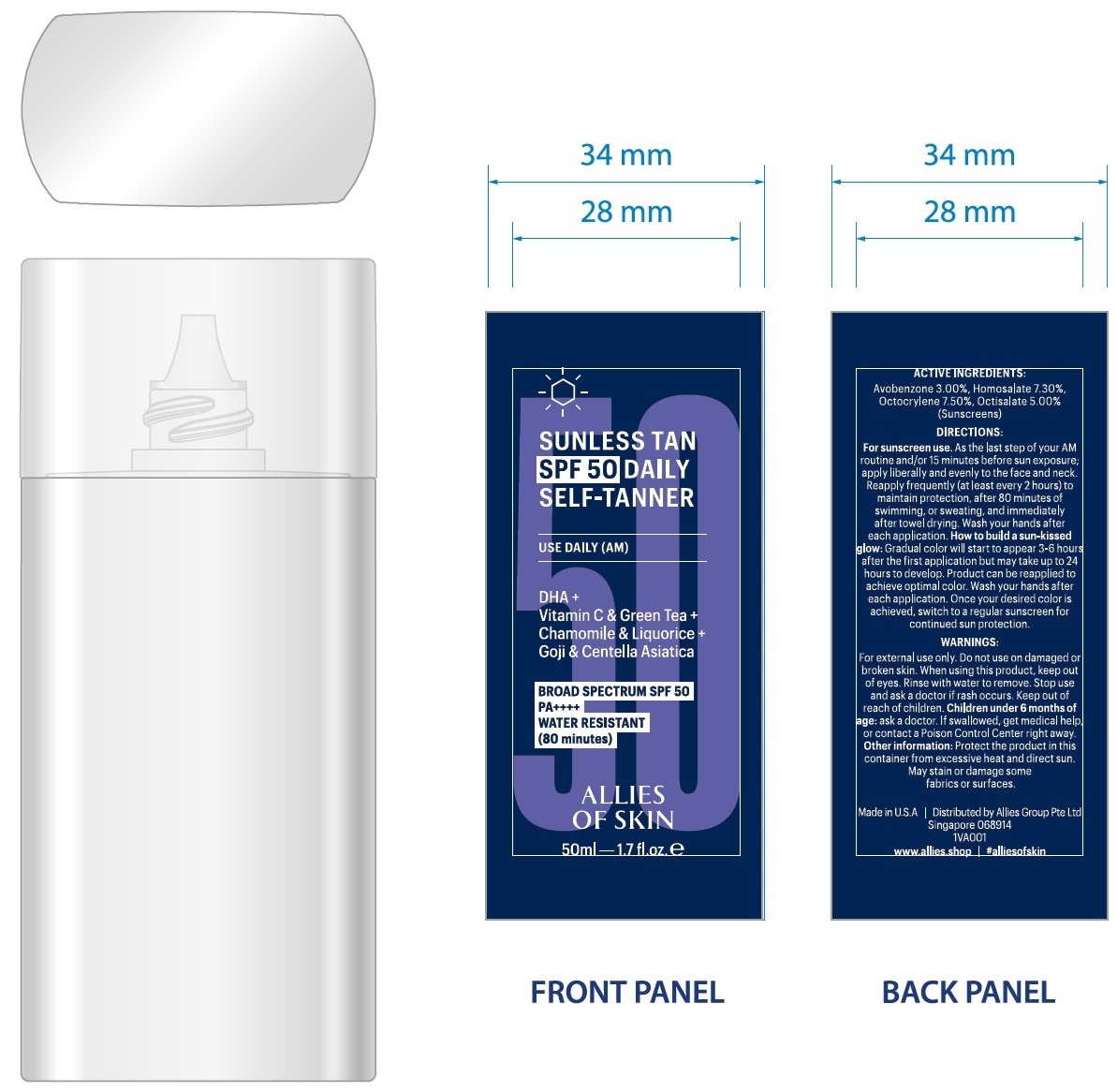
Label: ALLIES OF SKIN SUNLESS TAN SPF 50 DAILY SELF TANNER- avobenzone, homosalate, octocrylene, octisalate liquid
- NDC Code(s): 83852-543-00
- Packager: ALLIES GROUP PTE. LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses:
- Warnings:
-
Directions:
- Apply liberally 15 minutes before sun exposure and as needed.
- Children under 6 months of age: ask a doctor.
- Reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Other Information:
-
Inactive Ingredients:
Aloe Barbadensis Leaf Juice, Ascorbic Acid, Bisabolol, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, C9-12 Alkane, Camellia Sinensis Leaf Extract, Caprylic/Capric Triglyceride, Capryloyl Glycerin/Sebacic Acid Copolymer, Centella Asiatica Extract, Chamomilla Recutita (Matricaria) Flower Extract, Citric Acid, Daucus Carota Sativa (Carrot) Seed Extract, Dihydroxyacetone, Dimethicone/Bis-Isobutyl PPG-20 Crosspolymer, Dodecane, Ethyl Vanillin, Ethylhexylglycerin, Glycerin, Glyceryl Stearate Citrate, Glyceryl Stearate SE, Glycyrrhiza Glabra (Licorice) Root Extract, Helianthus Annuus (Sunflower) Seed Oil, Isododecane, Lactobacillus Ferment, Lycium Barbarum Fruit Extract, Magnesium Aluminum Silicate, Octyldodecyl Neopentanoate, Phenoxyethanol, Polysorbate 20, Potassium Cetyl Phosphate, Propanediol, Punica Granatum Fruit Extract, Sodium Hyaluronate, Sodium Metabisulfite, Sodium Stearoyl Glutamate, Tocopheryl Acetate, Vaccinium Angustifolium (Blueberry) Fruit Extract, Vitis Vinifera (Grape) Seed Oil, Water (Aqua), Xanthan Gum
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ALLIES OF SKIN SUNLESS TAN SPF 50 DAILY SELF TANNER
avobenzone, homosalate, octocrylene, octisalate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83852-543 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 73 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 75 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (ETHYLHEXYL SALICYLATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ASCORBIC ACID (UNII: PQ6CK8PD0R) LEVOMENOL (UNII: 24WE03BX2T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) C9-12 ALKANE (UNII: 7J5R5W72QM) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) CHAMOMILE (UNII: FGL3685T2X) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DAUCUS CAROTA SUBSP. SATIVUS SEED (UNII: 9M6AAX381U) DIHYDROXYACETONE (UNII: O10DDW6JOO) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) DODECANE (UNII: 11A386X1QH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) SUNFLOWER OIL (UNII: 3W1JG795YI) ISODODECANE (UNII: A8289P68Y2) LYCIUM BARBARUM FRUIT (UNII: 930626MWDL) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) PROPANEDIOL (UNII: 5965N8W85T) POMEGRANATE (UNII: 56687D1Z4D) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) GRAPE SEED OIL (UNII: 930MLC8XGG) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83852-543-00 1 in 1 BOX 10/14/2024 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/14/2024 Labeler - ALLIES GROUP PTE. LTD. (595420087)