AIROCARE- sodium fluoride liquid
Pharmacal-International Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warings
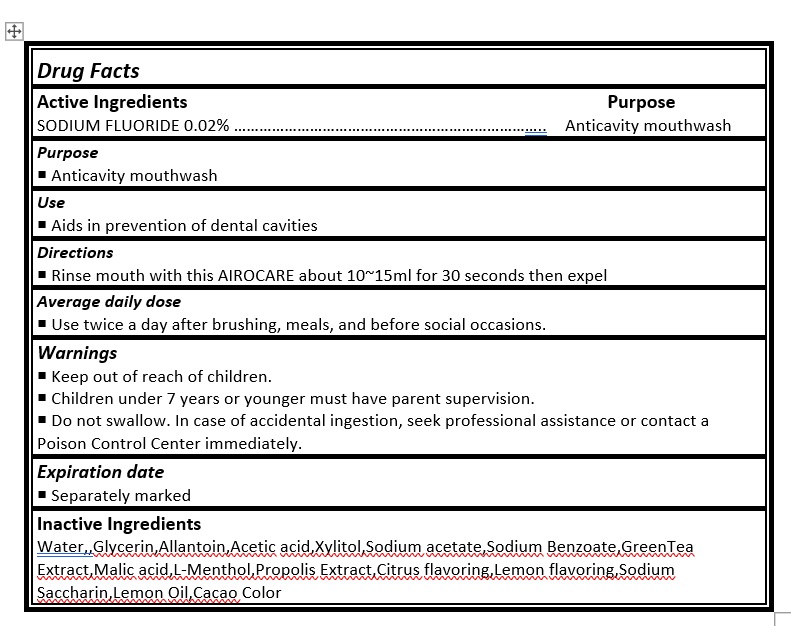
Do not swallow. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Uses
Aids in prevention of dental cavities. Use twice a day after brushing, meals, and before social occasions.
| AIROCARE
sodium fluoride liquid |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Pharmacal-International Co., Ltd (557805060) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ecoworld Co., Ltd | 688735061 | manufacture(24765-130) | |
Revised: 8/2023
Document Id: 021e3204-8e09-49f7-e063-6394a90a87cc
Set id: 4ce4c8e6-573a-40c9-966c-edbd0d0e0ae2
Version: 3
Effective Time: 20230804
Pharmacal-International Co., Ltd