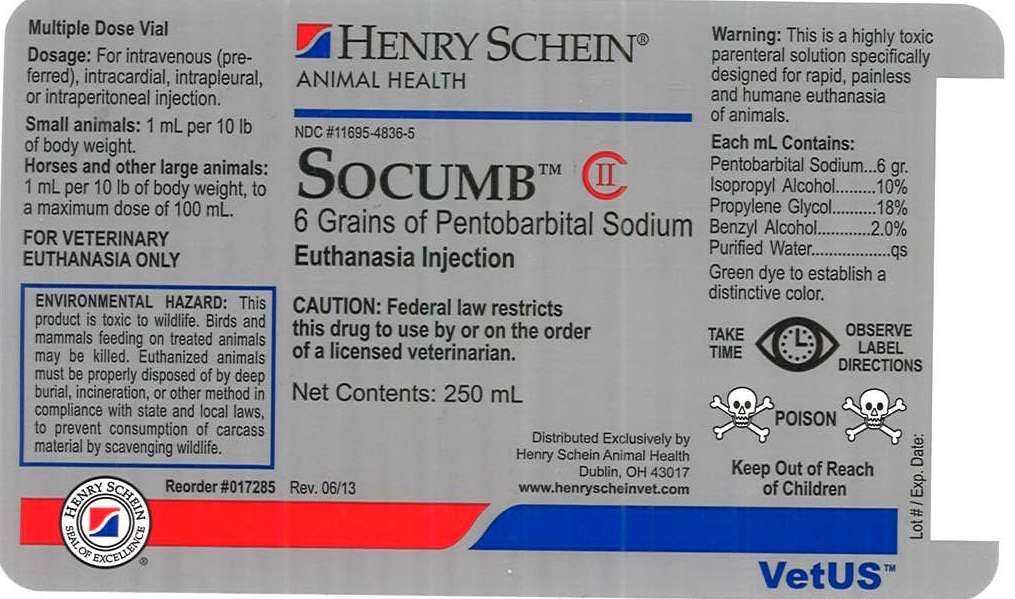
SOCUMB- euthanasia injection injection, solution
Henry Schein Animal Health
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
SOCUMB
DESCRIPTION
SOCUMB
6 Grains of Pentobarbital Sodium
Euthanasia Injection
Net Contents: 250 mL
FOR VETERINARY EUTHANASIA ONLY
Each mL Contains:
Pentobarbital Sodium...6 gr.
Isopropyl Alcohol.........10%
Propylene Glycol.........18%
Benzyl Alcohol............2.0%
Purified Water..............qs
Green dye to establish a distinctive color.
DOSAGE AND ADMINISTRATION
Multiple Dose Vial
Dosage: For intravenous (preferred), intracardial, intrapleural or intraperitoneal injection.
Small animals: 1 mL per 10 lb of body weight.
Horses and other large animals: 1 mL per 10 lb of body weight, to a maximum dose of 100 mL.
WARNING
This is a highly toxic parenteral solution specifically designed for rapid, painless and humane euthanasia of animals.
ENVIRONMENTAL HAZARD
This product is toxic to wildlife. Birds and mammals feeding on treated animals may be killed. Euthanized animals must be properly disposed of by deep burial, incineration, or other method in compliance with state and local laws, to prevent consumption of carcass material by scavenging wildlife.
| SOCUMB
euthanasia injection injection, solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Henry Schein Animal Health (603750329) |