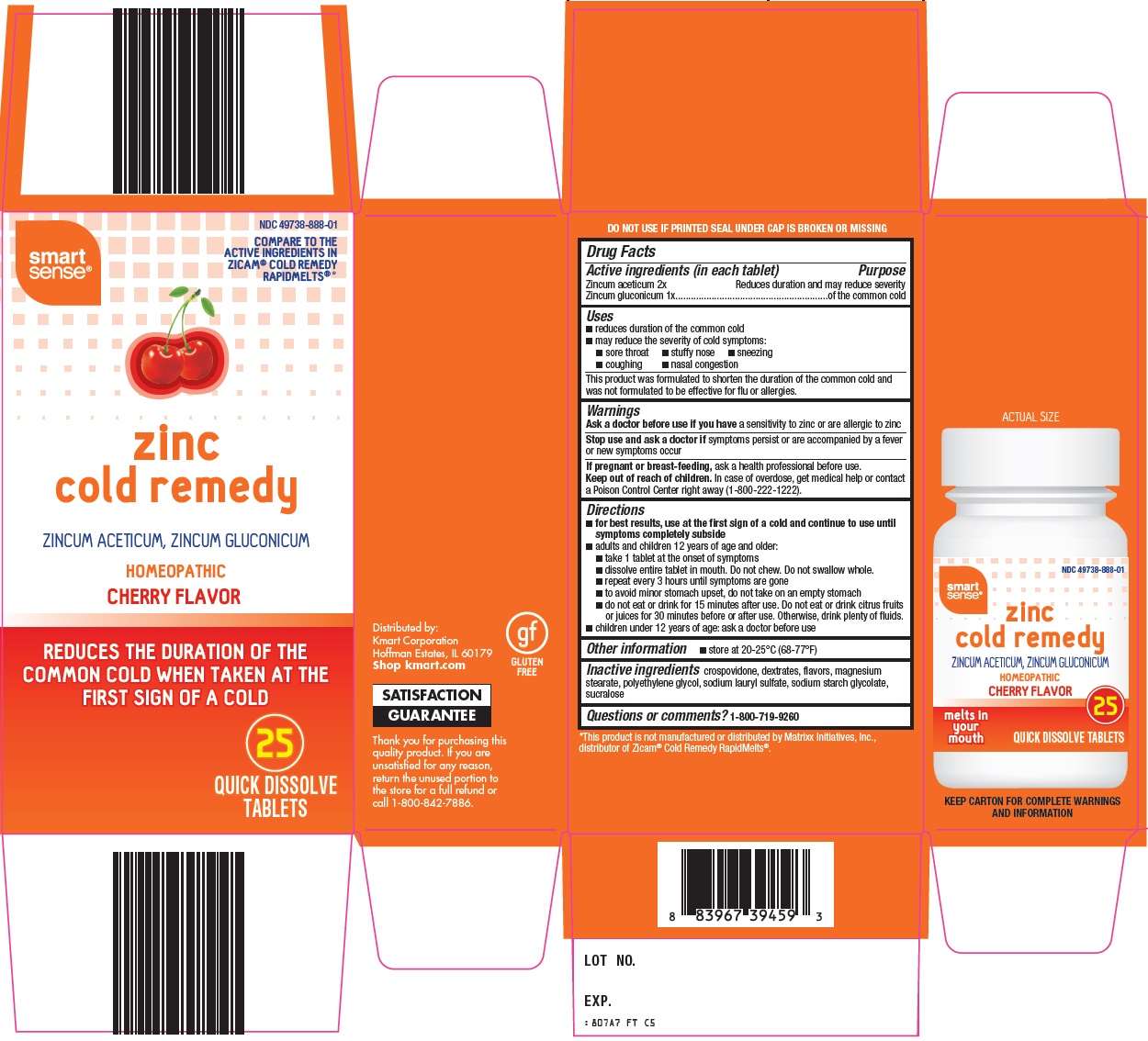
SMART SENSE ZINC COLD REMEDY- zincum aceticum, zincum gluconicum tablet, orally disintegrating
Kmart Corporation
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Kmart Corporation Zinc Cold Remedy Drug Facts
Uses
- •
- reduces duration of the common cold
- •
- may reduce the severity of cold symptoms:
- •
- sore throat
- •
- stuffy nose
- •
- sneezing
- •
- coughing
- •
- congestion
This product was formulated to shorten the duration of the common cold and was not formulated to be effective for flu or allergies.
Directions
- •
- for best results, use at the first sign of a cold and continue to use until symptoms completely subside
- •
- adults and children 12 years of age and older:
- •
- take 1 tablet at the onset of symptoms
- •
- dissolve entire tablet in mouth. Do not chew. Do not swallow whole.
- •
- repeat every 3 hours until symptoms are gone
- •
- to avoid minor stomach upset, do not take on an empty stomach
- •
- do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
- •
- children under 12 years of age: ask a doctor before use
| SMART SENSE ZINC COLD REMEDY
zincum aceticum, zincum gluconicum tablet, orally disintegrating |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kmart Corporation (008965873) |
Revised: 12/2020
Document Id: 76d1ec12-969e-491c-b560-fad94310c725
Set id: 4c3e7c07-fac4-491c-a6d3-43278bfac887
Version: 4
Effective Time: 20201229
Kmart Corporation