LORATADINE- loratadine tablet
Kaiser Foundation Hospitals
----------
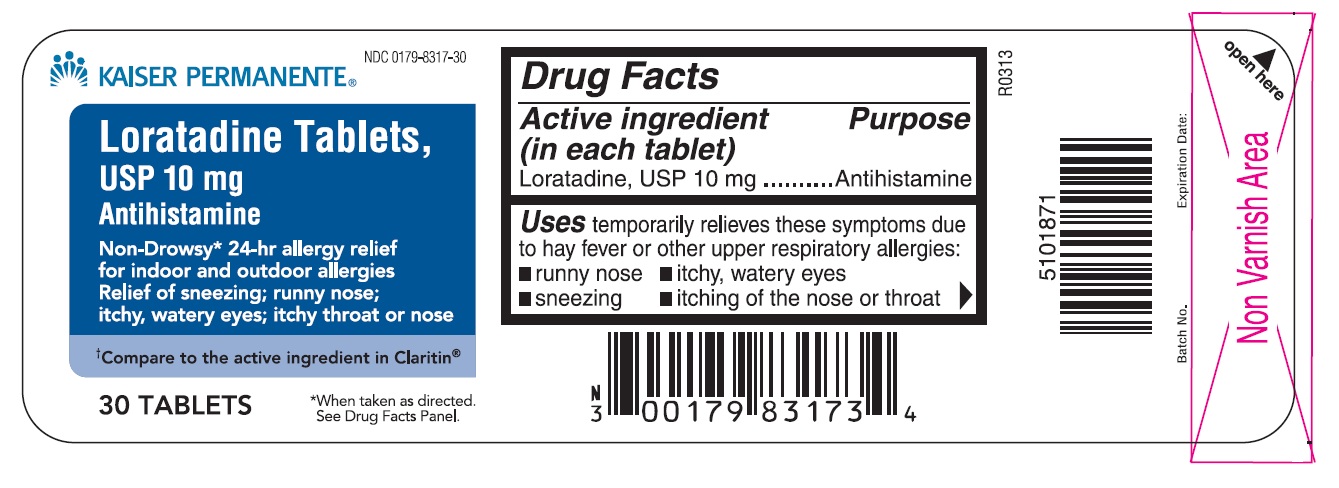
Drug Facts
USES
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
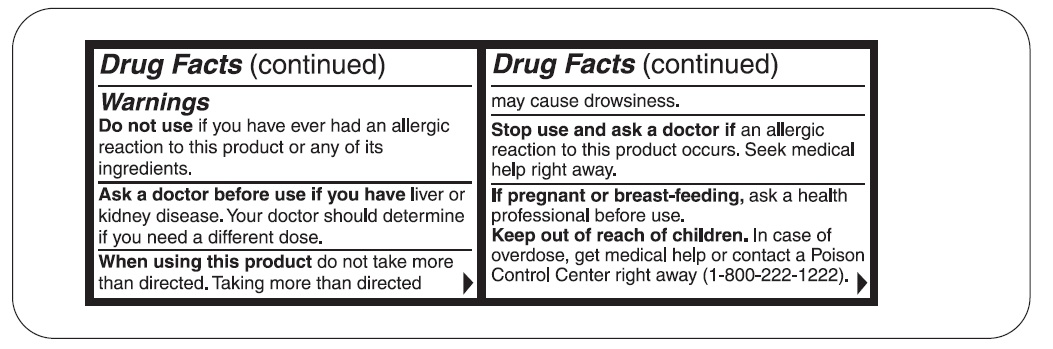
WARNINGS
Ask a doctor before use if you have
Liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
Do not take more than directed. Taking more than directed may cause drowsiness.
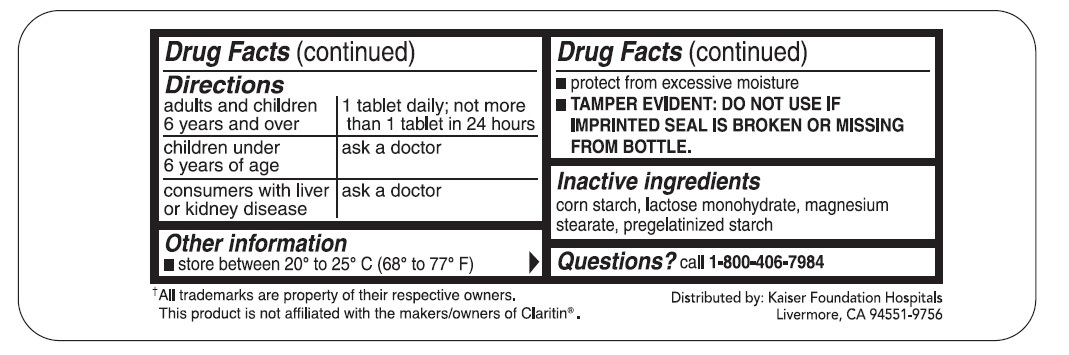
DIRECTIONS
| adults and children 6 years and over | 1 tablet daily; not more than 1 tablet in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
OTHER INFORMATION
- store between 20° to 25° C (68° to 77° F)
- protect from excessive moisture
- TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL IS BROKEN OR MISSING FROM BOTTLE.
PRINCIPAL DISPLAY PANEL
Non-Drowsy* 24-hr allergy relief
for indoor and outdoor allergies
Relief of sneezing; runny nose;
itchy, watery eyes; itchy throat or nose
†Compare to the active ingredient in Claritin ®
*When taken as directed. See Drug Facts Panel.
| LORATADINE
loratadine tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Kaiser Foundation Hospitals (053052619) |
| Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ohm Laboratories Inc. | 051565745 | manufacture(0179-8317) | |
Revised: 11/2018
Document Id: 7b098b92-2a83-569a-e053-2991aa0aae2d
Set id: 4ba788ce-d97e-442f-a6d6-c1843f86f4fe
Version: 2
Effective Time: 20181119
Kaiser Foundation Hospitals