Label: ALZAIR-
- NHRIC Code(s): 70914-030-01
- Packager: Hudson Scientific LLC
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Premarket Notification
Drug Label Information
Updated January 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Description
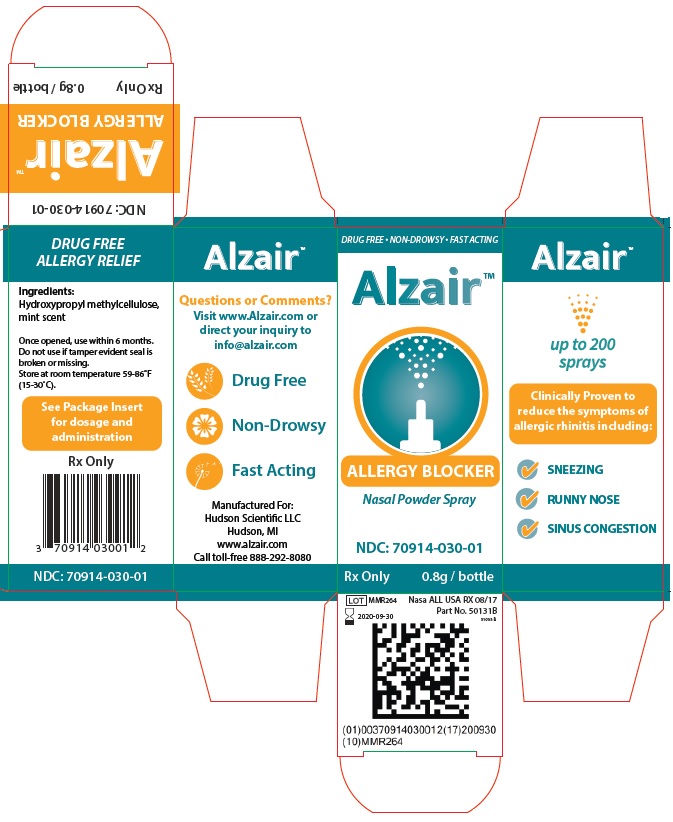
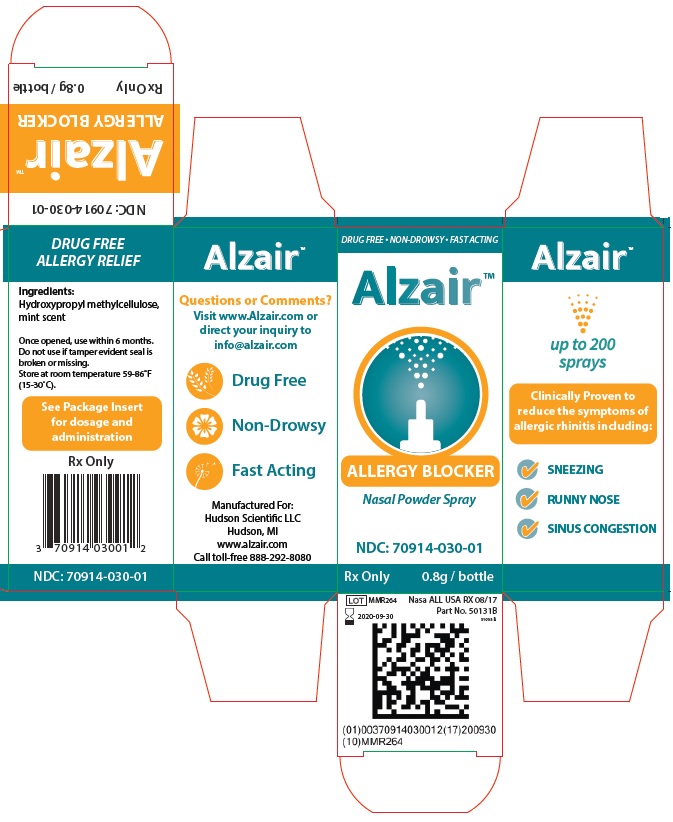
Alzair™ is a clinically proven, unique, natural product that works with the body's own nasal defense mechanism to strengthen its resistance to airborne allergens, such as pollen, dust mites, pet dander, and mold, which are inhaled through the nose. Alzair™ is a natural plant-based extract that meets the highest purity and safety standards.
Studies have shown that by naturally enhancing nasal mucus, it is possible to filter out airborn allergens. Alzair™ works by forming colorless, mucus-like gel lining in the nasal tract that acts as a barrier against pollen, dust, animal dander and mold.
Rx Only
-
Indications and Usage
Alzair™ Allergy Blocker is intended to treat hay fever and allergy sufferers by promoting alleviation of mild allergic symptoms (i.e. mild nasal irritation including itchy, runny, or congested nasal passages) triggered by the inhalation of various airborn allergens including indoor and outdoor environmental pollens, house dust, animal hairs and dust mites.
Application of Alzair™ produces a mucus-like gel barrier that evenly coats the nasal membranes and acts to block inhaled allergens within the nasal cavity.
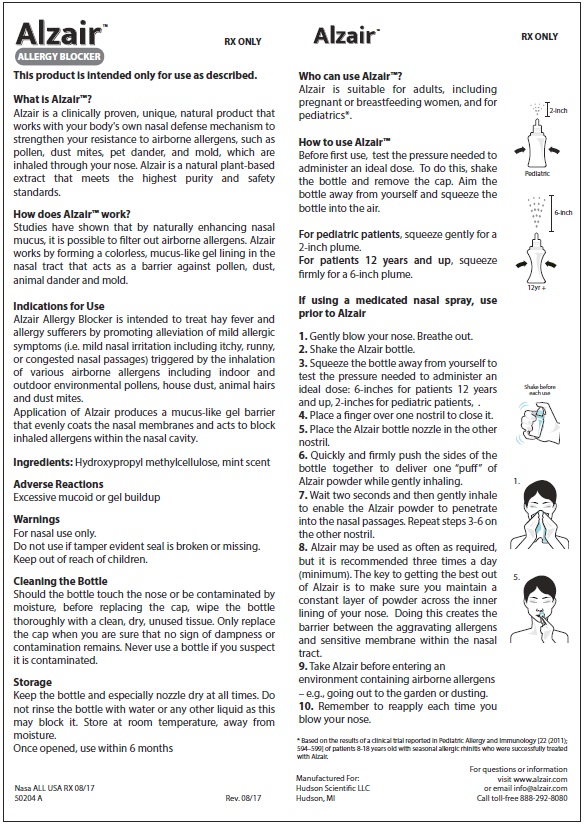
Who can use Alzair™:
Alzair™ is suitable for adults, including pregnant or breastfeeding women, and for pediatrics*.
Ingredients:
Hydroxypropyl methylcellulose, mint scent
- Warnings and Precautions:
-
Dosage & Administration:
Before first use, test the pressure needed to administer an ideal dose. To do this, shake the bottle and remove the cap. Aim the bottle away from yourself and squeeze the bottle into the air.
- For pediatric patients, squeeze gently for a 2-inch plume
- For patients 12 years and up, squeeze firmly for a 6-inch plume.
Shake bottle before each use.
Squeeze the bottle away from yourself. Test the pressure needed to administer an ideal dose, which is approximately a two-inch to six inch plume of powder (see above)
Gently blow your nose. Breath out.
Place a finger over one nostril to close it.
Place the Alzair™ bottle nozzle in the other nostril
Quickly and firmly push the sides of the bottle together to deliver one "puff" of Alzair™ powder while gently inhaling.
Wait two seconds and then gently inhale to enable the Alzair™ powder to penetrate into the nasal passages. Repeat steps 3-6 on the other nostril.Alzair™ may be used as often as required but is recommended three times a day (minimum). The key to getting the best out of Alzair™ is to make sure you maintain a constant layer of powder across the inner lining of your nose. Doing this creates the barrier between the aggravating allergens and sensitive membrane within the nasal tract.
Take Alzair™ before entering an environment containing airborne allergens- e.g. going out to the garden or dusting.
Remember to reapply each time you blow your nose.
* Based on the results of a clinical trial reported in Pediatric Allergy and Immunology [22 (2011); 594-599] of patients 8-18 years old with seasonal allergic rhinitis who were successfully treated with Alzair™.
- How Alzair™ is Supplied
- Manufacturer:
- Alzair™ Bottle Label:
- Alzair™ Insert
- Alzair™ Box
-
INGREDIENTS AND APPEARANCE
ALZAIR
cream, nasal, topical, mechanical allergen particle barrierProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:70914-030 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:70914-030-01 1 in 1 BOX 1 800 mg in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K170848 01/15/2021 Labeler - Hudson Scientific LLC (080339637) Establishment Name Address ID/FEI Business Operations Hudson Scientific LLC 080339637 relabel