FERROFORTE- iron dextran injection, solution
Vetoquinol N.-A. INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
FerroForte®
ACTIVE INGREDIENT: FerroForte® is a clear, free flowing dark brown solution containing a macromolecular dextran and iron complex containing 200 mg/mL elemental iron with 0.5% w/v phenol as a preservative.
DOSAGE AND ADMINISTRATION: Inject 1 mL (200 mg iron) intramuscularly at one (1) to three (3) days of age. Disinfect the site of injection.
Manufactured by:
Bimeda-MTC Animal Health Inc., Cambridge ON, N3C 2W4
Distributed by:
Vetoquinol N.-A. Inc.
2000, ch. Georges, Lavaltrie, QC, CANADA J5T 3S5
vetoquinol
Client Information Sheet
FerroForte®
FerroForte® is a sterile Iron Dextran for Injection (200 mg/mL) supplied in 100 mL bottles for veterinary use only. FerroForte® is not approved by the FDA.
FDA has agreed to allow Vetoquinol N.-A. Inc. to supply FerroForte® in order to help address the anticipated shortage of Iron Dextran Injection, 200 mg/mL for prevention and treatment of iron deficiency anemia in newborn piglets in the U.S.A.
FerroForte® is approved by Health Canada and exported to the U.S.A. by Vetoquinol N.-A. Inc.
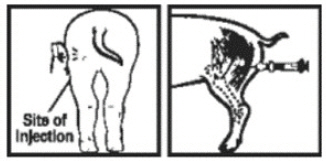
FerroForte® should be used according to the details of the Compendium text provided in the back of this Client Information Sheet. You can use FerroForte® as you would use any other Iron Dextran for Injection in the prevention and treatment of iron deficiency in newborn piglets in the U.S.A. For example, site of administration as illustrated below.
ADVERSE DRUG EVENTS & PRODUCT DEFECTS
To report suspected adverse drug events and product defects, please contact Vetoquinol N.-A.'s toll-free number at 1-800-363-1700 and inform the representative that your call concerns the Canadian product, FerroForte®. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
| FERROFORTE
iron dextran injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Vetoquinol N.-A. INC (249335886) |
| Registrant - Bimeda-MTC Animal Health (256232216) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Serumwerk Bernburg AG | 330105057 | API MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bimeda-MTC Animal Health | 256232216 | MANUFACTURE | |