Label: NASAL ALLERGY 24 HOUR- triamcinolone acetonide spray, metered
-
Contains inactivated NDC Code(s)
NDC Code(s): 50804-085-17 - Packager: Geiss, Destin & Dunn Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 19, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each spray)
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
Do not use
- ■
- in children under 2 years of age
- ■
- if you have ever had an allergic reaction to any of the ingredients
Ask a doctor before use if you
- ■
- have had recent nose ulcers or nose surgery
- ■
- have had a nose injury that has not healed
- ■
- are using a steroid medicine for asthma, allergies or skin rash
- ■
- have an eye infection
- ■
- have or had glaucoma or cataracts
When using this product
- ■
- the growth rate of some children may be slower
- ■
- some symptoms may get better on the first day of treatment. It may take up to one week of daily use to feel the most symptom relief.
- ■
- do not share this bottle with anyone else as this may spread germs
- ■
- remember to tell your doctor about all the medicines you take, including this one
Stop use and ask a doctor if
- ■
- you have, or come into contact with someone who has, chickenpox, measles or tuberculosis
- ■
- you have or develop symptoms of an infection such as a persistent fever
- ■
- you have any change in vision
- ■
- you have severe or frequent nosebleeds
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
-
Directions
Read insert (inside package) on how to:
- ■
- get a new bottle ready (primed) before first use
- ■
- prime bottle again if not used for more than 2 weeks
- ■
- use the spray
- ■
- clean the spray nozzle
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER
adults and children 12 years of age and older
- ■
- once daily, spray 2 times into each nostril while sniffing gently
- ■
- once your allergy symptoms improve, reduce to 1 spray in each nostril per day
CHILDREN 2 TO UNDER 12 YEARS OF AGE
- ■
- the growth rate of some children may be slower while using this product. Talk to your child’s doctor if your child needs to use the spray for longer than two months a year.
children 6 to under 12 years of age
- ■
- an adult should supervise use
- ■
- once daily, spray 1 time into each nostril while sniffing gently
- ■
- if allergy symptoms do not improve, increase to 2 sprays in each nostril per day. Once allergy symptoms improve, reduce to 1 spray in each nostril per day.
children 2 to under 6 years of age
- ■
- an adult should supervise use
- ■
- once daily, spray 1 time into each nostril while sniffing gently
children under 2 years of age
- ■
- do not use
- ■
- do not use more than directed
- ■
- if you forget a dose, do not double the next dose
- ■
- do not spray into eyes or mouth
- ■
- if allergy symptoms do not improve after one week, stop using and talk to a doctor
- ■
- do not use for the common cold
- ■
- shake well before each use
- Other information
- Inactive ingredients
- Questions or comments?
-
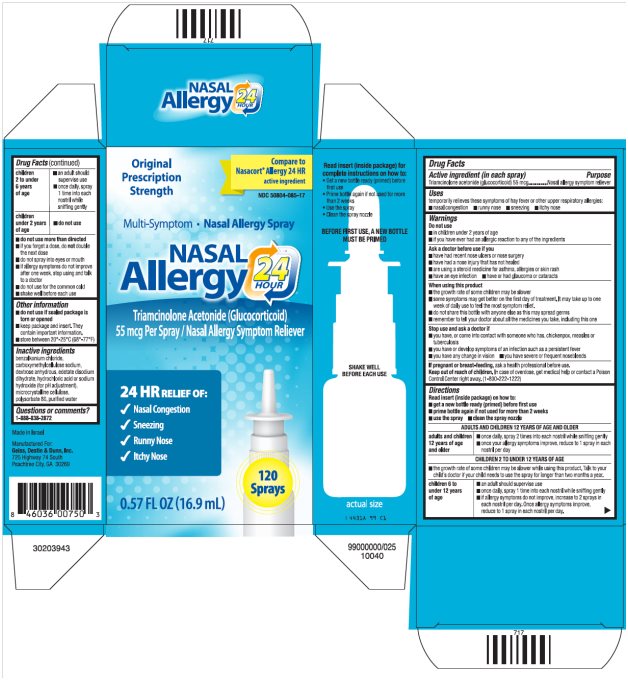
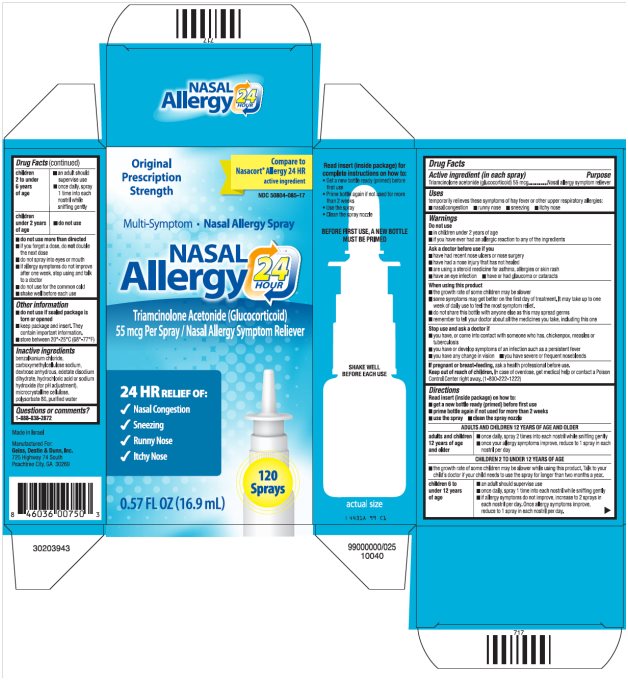
Package/Label Principal Display Panel
NASAL Allergy 24 HOUR Carton Text
Original Prescription Strength
Compare to Nasacort® Allergy 24 HR active ingredient
NDC 50804-085-17
Multi-Symptom ·Nasal Allergy Spray
NASAL
Allergy 24 HOUR
Triamcinolone Acetonide (Glucocorticoid)
55 mcg Per Spray/Nasal Allergy Symptom Reliever
24 HR RELIEF OF:
- Nasal Congestion
- Sneezing
- Runny Nose
- Itchy Nose
120 Sprays
0.57 FL OZ (16.9 mL)
-
INGREDIENTS AND APPEARANCE
NASAL ALLERGY 24 HOUR
triamcinolone acetonide spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50804-085 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIAMCINOLONE ACETONIDE (UNII: F446C597KA) (TRIAMCINOLONE ACETONIDE - UNII:F446C597KA) TRIAMCINOLONE ACETONIDE 55 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50804-085-17 1 in 1 CARTON 1 120 in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078104 06/19/2015 Labeler - Geiss, Destin & Dunn Inc. (076059836) Registrant - Teva Pharmaceuticals USA Inc (001627975)