Label: ZFLEX- acetaminophen and phenyltoloxamine citrate tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 35501-023-01 - Packager: Huckaby Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 12, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each Tablet Contains:
Acetaminophen 500 mg 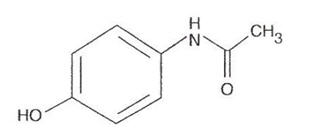
Phenyltoloxamine Citrate 55 mg 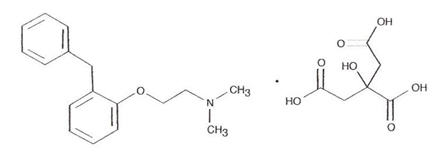
Acetaminophen, 4'-hydroxyacetanilide, a slightly bitter, white odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic.
Phenyltoloxamine citrate is an antihistamine having the chemical name N, N-dimethyl-2-(α-phenyl-o-tolyloxy) ethylamine dihydrogen citrate.
Inactive ingredients: Each tablet contains povidone, microcrystalline cellulose, sodium starch glycolate and magnesium stearate.
-
CLINICAL PHARMACOLOGY
The analgesic action of acetaminophen involves peripheral influences, but the specific mechanism is as yet undetermined. Antipyretic activity is mediated through hypothalamic heat regulating centers. Acetaminophen inhibits prostaglandin synthetase. Therapeutic doses of acetaminophen have negligible effects on the cardiovascular or respiratory systems; however, toxic doses may cause circulatory failure and rapid, shallow breathing.
Pharmacokinetics
The behavior of the individual components is described below.
Acetaminophen
Acetaminophen is rapidly absorbed from the gastrointestinal tract and is distributed throughout most body tissues. The plasma half-life is 1.25 to 3 hours, but may be increased by liver damage and following overdosage. Elimination of acetaminophen is principally by liver metabolism (conjugation) and subsequent renal excretion of metabolites. Approximately 85% of an oral dose appears in the urine within 24 hours of administration, most as the glucuronide conjugate, with small amounts of other conjugates and unchanged drug.
See OVERDOSAGE for toxicity information.
Phenyltoloxamine Citrate
Phenyltoloxamine is an H1 blocking agent which interferes with the action of histamine primarily in capillaries surrounding mucous tissues and sensory nerves of the nasal and adjacent areas. It has the ability to interfere with certain areas of acetylcholine-inhibiting secretions in the nose, mouth and pharynx. It commonly causes CNS depression.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
Laboratory Tests
In patients with severe hepatic or renal disease, effects of therapy should be monitored with serial liver and/or renal function tests.
Drug/Laboratory Test Interactions
Acetaminophen may produce false-positive test results for urinary 5-hydroxyindoleacetic acid. The sedative effects of phenyltoloxamine citrate are additive to the CNS depressant effects of alcohol, hypnotics, sedatives and tranquilizers.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate studies have been conducted in animals to determine whether phenyltoloxamine citrate or acetaminophen has a potential for carcinogenesis, mutagenesis, or impairment of fertility.
Nursing Mothers
Acetaminophen is excreted in breast milk in small amounts, but the significance of its effects on nursing infants is not known. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from phenyltoloxamine citrate and acetaminophen, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
- ADVERSE REACTIONS
-
OVERDOSAGE
Following an acute overdosage, toxicity may result from phenyltoloxamine citrate or acetaminophen.
Acetaminophen
In acetaminophen overdosage: dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia may also occur.
Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
In adults, hepatic toxicity has rarely been reported with acute overdoses of less than 10 grams, or fatalities with less than 15 grams.
Treatment
A single or multiple overdose with phenyltoloxamine citrate or acetaminophen is a potentially lethal overdose, and consultation with a regional poison control center is recommended. Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Vomiting should be induced mechanically, or with syrup of ipecac, if the patient is alert (adequate pharyngeal and laryngeal reflexes).
If the dose of acetaminophen may have exceeded 140 mg/kg, acetylcysteine should be administered as early as possible. Serum acetaminophen levels should be obtained, since levels four or more hours following ingestion help predict acetaminophen toxicity. Do not await acetaminophen assay results before initiating treatment. Hepatic enzymes should be obtained initially, and repeated at 24-hour intervals. Methemoglobinemia over 30% should be treated with methylene blue by slow intravenous administration. The toxic dose for adults for acetaminophen is 10 g.
- Dosage
-
WARNINGS
Do not take this product for pain for more than 10 days (adults) or 5 days (children), and do not take for fever for more than 3 days unless directed by a physician. If pain or fever persists, if new symptoms occur, or if redness or swelling is present, consult a physician immediately because these could be signs of a serious condition. Do not give this product to children under 12 years of age for the pain of arthritis unless directed by a physician. May cause drowsiness; alcohol, sedatives and tranquilizers may increase the drowsiness effect. Avoid alcoholic beverages while taking this product. Do not take this product if you are taking sedatives or tranquilizers without first consulting your physician. Use caution when driving a motor vehicle or operating machinery.
Keep this and all drugs out of the reach of children. In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately. Prompt medical attention is critical, for adults as well as for children, even if you do not notice any signs or symptoms. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product.
- STORAGE AND HANDLING
-
HOW SUPPLIED
NDC# 35501-023-01 Bottles of 100 count tablets; and samples NDC# 35501-023-02 containing 4 tablets. A red capsule shaped tablet with a score on both sides. Both sides with "ZFLEX" on the left side of the score and a blank space on the right side of the score. If tablet is broken at the score, one side will display "ZFLEX" and the other side will be blank.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
ZFLEX
acetaminophen and phenyltoloxamine citrate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:35501-023 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Acetaminophen (UNII: 362O9ITL9D) (Acetaminophen - UNII:362O9ITL9D) Acetaminophen 500 mg Phenyltoloxamine Citrate (UNII: 8UE48MJH8M) (Phenyltoloxamine - UNII:K65LB6598J) Phenyltoloxamine Citrate 55 mg Inactive Ingredients Ingredient Name Strength povidone (UNII: FZ989GH94E) cellulose, microcrystalline (UNII: OP1R32D61U) magnesium stearate (UNII: 70097M6I30) Product Characteristics Color RED Score 2 pieces Shape RECTANGLE Size 19mm Flavor Imprint Code ZFLEX Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35501-023-01 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 04/12/2010 Labeler - Huckaby Pharmaceuticals, Inc. (624844507)


