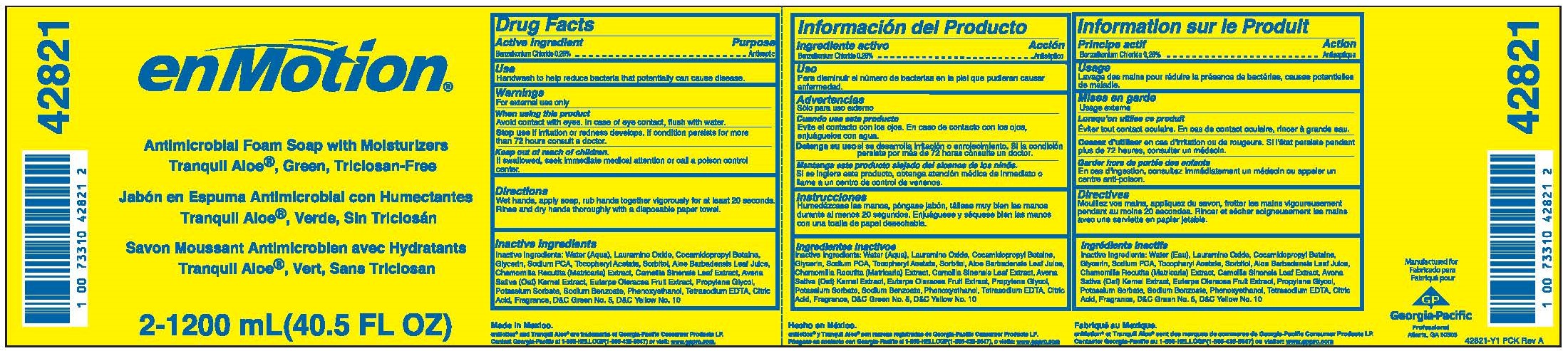
ENMOTION ANTIMICROBIAL FOAM WITH MOISTURIZERS TRANQUIL ALOE, GREEN TRICLOSAN-FREE- benzalkonium chloride solution
Georgia-Pacific Consumer Products
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Benzalkonium chloride, 0.26% w/w
Use
Handwash to help reduce bacteria that potentially can cause disease.
Warnings
When using this product
Avoid contact with eyes. In case of eye contact, flush with water.
Stop use
if irritation or redness develops. If condition persists for more than 72 hours consult a doctor.
Keep out of reach of children.
If swallowed, seek immediate medical attention or call a poison control center.
Directions
Wet hands, apply soap, rub hands together vigorously for at least 20 seconds.
Rinse and dry hands thoroughly with a disposable paper towel.
Inactive ingredients
Water (Aqua), Lauramine Oxide, Cocamidopropyl Betaine, Glycerin, Sodium PCA, Tocopheryl Acetate, Sorbitol, Aloe Barbadensis Leaf Juice, Chamomilla Recutita (Matricaria) Extract, Camellia Sinensis Leaf Extract, Avena Sativa (Oat) Kernel Extract, Euterpe Oleracea Fruit Extract, Propylene Glycol, Potassium Sorbate, Sodium Benzoate, Phenoxyethanol, Tetrasodium EDTA, Citric Acid, Fragrance, D&C Green No. 5, D&C Yellow No. 10.
enMotion
Antimicrobial Foam Soap With Moisturizers
Tranquil Aloe, Green, Triclosan-Free
Net Contents 2-1200mL (40.5 FL OZ)
Made in Mexico
enMotion is a trademark of Georgia-Pacific Consumer Products LP.
Contact Georgia-Pacific at 1-866-HELLOGP(1-866-435-5647)