Label: ANTISEPTIC SKIN CLEANSER- chlorhexidine gluconate 4% liquid
- NDC Code(s): 0116-0161-08, 0116-0161-15, 0116-0161-16
- Packager: Xttrium Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 9, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
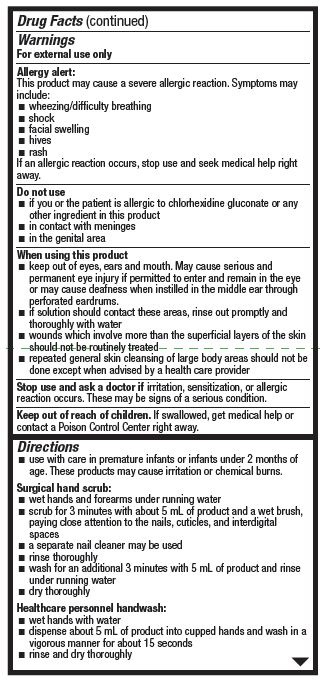
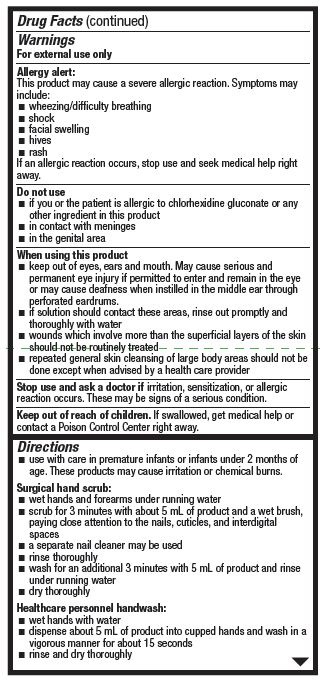
Warnings
For external use only
Allergy alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do not use
- if you or the patient is allergic to chlorhexidine gluconate or any other ingredient in this product
- in contact with meninges
- in the genital area
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if pemitted to ener and remain in the eye or may cause deafness when instilled in the middle eat through perforated eardrums..
- if solution should contact these areas, rinse out promptly and thoroughly with water
- wounds which involve more than the superficial layers of the skin should not be routinely treated
- repeated general skin cleansing of large body areas should not be done except when advised by a health care provider
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand scrub:
- wet hands and forearms under running water
- scrub for 3 minutes with about 5 mL of product and a wet brush, paying close attention to the nails, cuticles, and interdigital spaces
- a separate nail cleaner may be used
- rinse thoroughly
- wash for an additional 3 minutes with 5 mL of product and rinse under running water
- dry thoroughly
Healthcare personnel handwash:
- wet hands with water
- dispense about 5 mL of product into cupped hands and wash in a vigorous manner for about 15 seconds
- rinse and dry thoroughly
Skin wound and normal skin cleansing:
- thoroughly rinse the area to be cleaned with water
- apply the minimum amount of product necessary to cover the skin or wound area and wash gently
- rinse again thoroughly
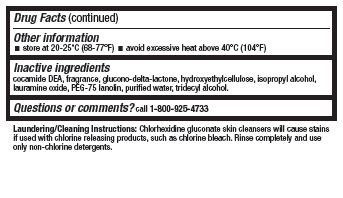
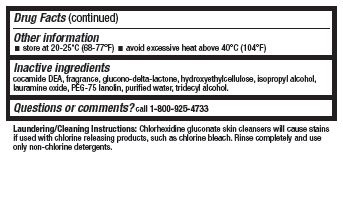
- Other information
- Inactive ingredients
- Questions or comments?
-
OTHER SAFETY INFORMATION
Laundering/Cleaning Instructions: Chlorhexidine gluconate skin cleansers will cause stains if used with chlorine-releasing products. Rinse completely and use only non-chlorine detergents.
WARNING: This product contains a chemical (cocamide DEA) known to the State of California to cause cancer.
‡Walgreens Pharmacist Survey Study, November 2014
‡‡This product is not manufactured or distributed by Mölnlycke Healthcare, owner of the registered trademark Hibiclens®.
Distributed by: Walgreen Co.
200 Wilmot RD., Deerfield, IL 60015
100% Satisfaction Guaranteed
walgreens.com
©2015 Walgreen Co. Made in USA
-
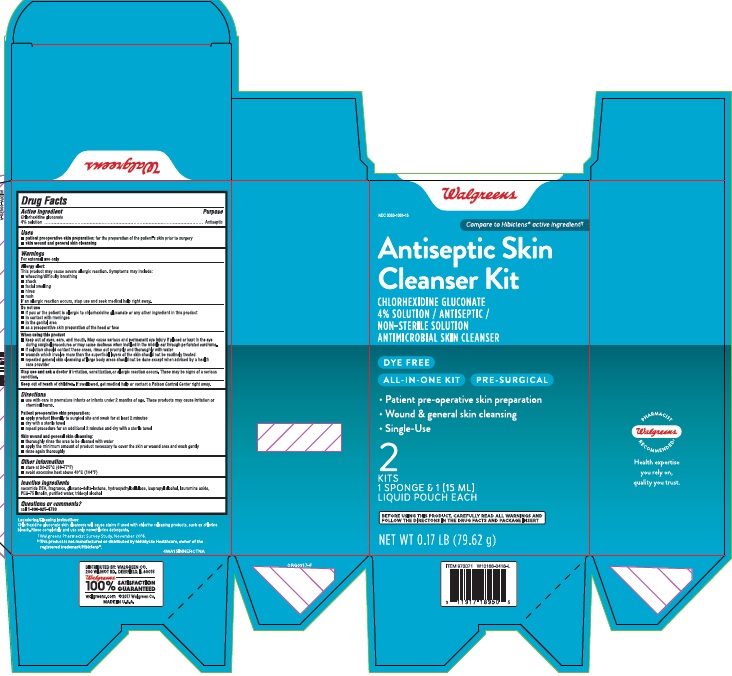
Package label and principal display panel
Walgreens
Compare to the Hibiclens® active ingredient
NDC 0363-1061-08
Dye-Free
Antiseptic Skin Cleanser
CHLORHEXIDINE GLUCONATE 4% SOLUTION / ANTISEPTIC / ANTIMICROBIAL SKIN CLEANSER
DYE FREE
TAMPER EVIDENT: DO NOT USE IF SAFETY SEAL IS BROKEN OR MISSING
8 FL OZ (237 mL)



VALUE SIZE
NDC 0363-1061-16
Walgreens
Compare to Hibiclens® active ingredient
Dye-Free Antiseptic Skin Cleanser
CHLORHEXIDINE GLUCONATE 4% SOLUTION / ANTISEPTIC / ANTIMICROBIAL SKIN CLEANSER
DYE FREE
Skin wound & general skin cleansing
TAMPER EVIDENT: DO NOT USE IF SAFETY SEAL IS BROKEN OR MISSING
16 FL OZ (473 mL)



- Carton
- Lidstock
- Packet
-
INGREDIENTS AND APPEARANCE
ANTISEPTIC SKIN CLEANSER
chlorhexidine gluconate 4% liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0116-0161 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 4 mg in 100 mL Inactive Ingredients Ingredient Name Strength LAURAMINE OXIDE (UNII: 4F6FC4MI8W) COCO DIETHANOLAMIDE (UNII: 92005F972D) PEG-75 LANOLIN (UNII: 09179OX7TB) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) TRIDECYL ALCOHOL (UNII: 8I9428H868) GLUCONOLACTONE (UNII: WQ29KQ9POT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0116-0161-08 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/01/2011 2 NDC:0116-0161-16 473 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2011 3 NDC:0116-0161-15 15 mL in 1 KIT; Type 1: Convenience Kit of Co-Package 01/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019125 05/01/2011 Labeler - Xttrium Laboratories, Inc. (007470579) Registrant - Xttrium Laboratories, Inc. (007470579) Establishment Name Address ID/FEI Business Operations Xttrium Laboratories, Inc. 007470579 manufacture(0116-0161)