Label: MEPROBAMATE tablet
-
NDC Code(s):
51672-4147-1,
51672-4147-3,
51672-4147-6,
51672-4148-1, view more51672-4148-3, 51672-4148-6
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated January 15, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Meprobamate is a white powder with a characteristic odor and a bitter taste. It is slightly soluble in water, freely soluble in acetone and alcohol, and sparingly soluble in ether. The structural formula of meprobamate is:
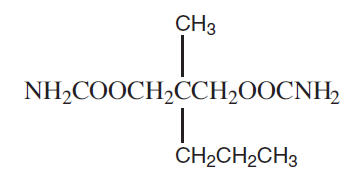
C9H18N2O4 M.W. 218.25
Meprobamate Tablets USP, 200 mg and 400 mg for oral administration contain the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, and stearic acid.
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
Meprobamate tablets are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually do not require treatment with an anxiolytic.
The effectiveness of meprobamate tablets in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
- CONTRAINDICATIONS
-
WARNINGS
Drug Dependence
Physical dependence, psychological dependence, and abuse have occurred. When chronic intoxication from prolonged use occurs, it usually involves ingestion of greater than recommended doses and is manifested by ataxia, slurred speech, and vertigo. Therefore, careful supervision of dose and amounts prescribed is advised, as well as avoidance of prolonged administration, especially for alcoholics and other patients with a known propensity for taking excessive quantities of drugs.
Sudden withdrawal of the drug after prolonged and excessive use may precipitate recurrence of pre-existing symptoms such as anxiety, anorexia, insomnia, or withdrawal reactions such as vomiting, ataxia, tremors, muscle twitching, confusional states, hallucinosis, and rarely, convulsive seizures. Such seizures are more likely to occur in persons with central nervous system damage or pre-existent or latent convulsive disorders. Onset of withdrawal symptoms occurs usually within 12 to 48 hours after discontinuation of meprobamate; symptoms usually cease within the next 12 to 48 hours.
When excessive dosage has continued for weeks or months, dosage should be reduced gradually over a period of one or two weeks rather than abruptly stopped. Alternatively, a long-acting barbiturate may be substituted, then gradually withdrawn.
Potentially Hazardous Tasks
Patients should be warned that meprobamate may impair the mental and/or physical abilities required for performance of potentially hazardous tasks such as driving or operating machinery.
Additive Effects
Since the effects of meprobamate and alcohol or meprobamate and other CNS depressants or psychotropic drugs may be additive, appropriate caution should be exercised with patients who take more than one of these agents simultaneously.
Usage in Pregnancy and Lactation
An increased risk of congenital malformations associated with the use of minor tranquilizers (meprobamate, chlordiazepoxide and diazepam) during the first trimester of pregnancy has been suggested in several studies. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physician about the desirability of discontinuing the drug.
Meprobamate passes the placental barrier. It is present both in umbilical cord blood at or near maternal plasma levels and in breast milk of lactating mothers at concentrations two to four times that of maternal plasma. When use of meprobamate is contemplated in breastfeeding patients, the drug's higher concentration in breast milk as compared to maternal plasma should be considered.
-
PRECAUTIONS
The lowest effective dose should be administered, particularly to elderly and/or debilitated patients, in order to preclude oversedation. The possibility of suicide attempts should be considered and the least amount of drug feasible should be dispensed at any one time. Meprobamate is metabolized in the liver and excreted by the kidney; to avoid its excess accumulation, caution should be exercised in administration to patients with compromised liver or kidney function.
Meprobamate occasionally may precipitate seizures in epileptic patients.
Geriatric use
Clinical studies of meprobamate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Central Nervous System
Drowsiness, ataxia, dizziness, slurred speech, headache, vertigo, weakness, paresthesias, impairment of visual accommodation, euphoria, overstimulation, paradoxical excitement, fast EEG activity.
Cardiovascular
Palpitation, tachycardia, various forms of arrhythmia, transient ECG changes, syncope; also hypotensive crisis (including one fatal case).
Allergic or Idiosyncratic
Allergic or idiosyncratic reactions are usually seen within the period of the first to fourth dose in patients having had no previous contact with the drug. Milder reactions are characterized by an itchy, urticarial, or erythematous maculopapular rash which may be generalized or confined to the groin. Other reactions have included leukopenia, acute nonthrombocytopenic purpura, petechiae, ecchymoses, eosinophilia, peripheral edema, adenopathy, fever, fixed drug eruption with cross reaction to carisoprodol, and cross sensitivity between meprobamate/mebutamate and meprobamate/carbromal.
More severe hypersensitivity reactions, rarely reported, include hyperpyrexia, chills, angioneurotic edema, bronchospasm, oliguria and anuria. Also, anaphylaxis, erythema multiforme, exfoliative dermatitis, stomatitis, proctitis, Stevens-Johnson syndrome and bullous dermatitis, including one fatal case of the latter following administration of meprobamate in combination with prednisolone.
In case of allergic or idiosyncratic reactions to meprobamate, discontinue the drug and initiate appropriate symptomatic therapy, which may include epinephrine, antihistamines, and in severe cases, corticosteroids. In evaluating possible allergic reactions, also consider allergy to excipients.
Hematologic
(See also Allergic or Idiosyncratic.) Agranulocytosis and aplastic anemia have been reported. These cases rarely were fatal. Rare cases of thrombocytopenic purpura have been reported.
-
OVERDOSAGE
Suicidal attempts with meprobamate have resulted in drowsiness, lethargy, stupor, ataxia, coma, shock, vasomotor, and respiratory collapse. Some suicidal attempts have been fatal.
The following data on meprobamate tablets have been reported in the literature and from other sources. These data are not expected to correlate with each case (considering factors such as individual susceptibility and length of time from ingestion to treatment), but represent the usual ranges reported.
Acute simple overdose (meprobamate alone): Death has been reported with ingestion of as little as 12 g meprobamate and survival with as much as 40 g.
Blood Levels
0.5-2 mg% represents the usual blood level range of meprobamate after therapeutic doses. The level may occasionally be as high as 3 mg%.
3-10 mg% usually corresponds to findings of mild to moderate symptoms of overdosage, such as stupor or light coma.
10-20 mg% usually corresponds to deeper coma, requiring more intensive treatment. Some fatalities occur.
At levels greater than 20 mg%, more fatalities than survivals can be expected.
Acute combined overdose (meprobamate with alcohol or other CNS depressants or psychotropic drugs): Since effects can be additive, a history of ingestion of a low dose of meprobamate plus any of these compounds (or of a relative low blood or tissue level) cannot be used as a prognostic indicator.
In cases where excessive doses have been taken, sleep ensues rapidly and blood pressure, pulse, and respiratory rates are reduced to basal levels. Any drug remaining in the stomach should be removed and symptomatic therapy given. Should respiration or blood pressure become compromised, respiratory assistance, central nervous system stimulants, and pressor agents should be administered cautiously as indicated. Meprobamate is metabolized in the liver and excreted by the kidney. Diuresis, osmotic (mannitol) diuresis, peritoneal dialysis, and hemodialysis have been used successfully. Careful monitoring of urinary output is necessary and caution should be taken to avoid overhydration. Relapse and death, after initial recovery, have been attributed to incomplete gastric emptying and delayed absorption. Meprobamate can be measured in biological fluids by two methods: colorimetric (Hoffman, A.J. and Ludwig, B.J.: J Amer Pharm Assn 48: 740, 1959) and gas chromatographic (Douglas, J.F. et al: Anal Chem 39: 956, 1967).
-
DOSAGE AND ADMINISTRATION
Meprobamate Tablets USP
The usual adult daily dosage is 1200 mg to 1600 mg, in three or four divided doses; a daily dosage above 2400 mg is not recommended. The usual daily dosage for children ages six to twelve years is 200 mg to 600 mg, in two or three divided doses.
Not recommended for children under age 6 (see Usage in Children).
-
HOW SUPPLIED
Meprobamate Tablets USP, 200 mg are white, round tablets, scored on one side. Engraved "T" above the score and "M2" below the score line. Plain on the other side.
Bottle of 30 NDC 51672-4147-6 Bottle of 100 NDC 51672-4147-1 Bottle of 1000 NDC 51672-4147-3 Meprobamate Tablets USP, 400 mg are white, round tablets, scored on one side. Engraved "T" above the score and "M4" below the score line. Plain on the other side.
Bottle of 30 NDC 51672-4148-6 Bottle of 100 NDC 51672-4148-1 Bottle of 1000 NDC 51672-4148-3 - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 400 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
MEPROBAMATE
meprobamate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51672-4147 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Meprobamate (UNII: 9I7LNY769Q) (Meprobamate - UNII:9I7LNY769Q) Meprobamate 200 mg Inactive Ingredients Ingredient Name Strength silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) sodium starch glycolate type a potato (UNII: 5856J3G2A2) stearic acid (UNII: 4ELV7Z65AP) starch, corn (UNII: O8232NY3SJ) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 9mm Flavor Imprint Code T;M2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51672-4147-6 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2011 2 NDC:51672-4147-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2011 3 NDC:51672-4147-3 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200998 05/23/2011 MEPROBAMATE
meprobamate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51672-4148 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Meprobamate (UNII: 9I7LNY769Q) (Meprobamate - UNII:9I7LNY769Q) Meprobamate 400 mg Inactive Ingredients Ingredient Name Strength silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) sodium starch glycolate type a potato (UNII: 5856J3G2A2) stearic acid (UNII: 4ELV7Z65AP) starch, corn (UNII: O8232NY3SJ) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 11mm Flavor Imprint Code T;M4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51672-4148-6 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2011 2 NDC:51672-4148-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2011 3 NDC:51672-4148-3 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/23/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200998 05/23/2011 Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceutical Industries Ltd. 600072078 MANUFACTURE(51672-4147, 51672-4148)




