MAXIMUM STRENGTH RANITIDINE - ranitidine tablet
Aurohealth LLC
----------
Tips for managing heartburn
- Do not lie flat or bend over soon after eating
- Do not eat late at night, or just before bedtime
- Certain foods or drinks are more likely to cause heartburn, such as rich, spicy, fatty, and fried foods, chocolate, caffeine, alcohol, even some fruits and vegetables
- Eat slowly and do not eat big meals
- If you are overweight, lose weight
- If you smoke, quit smoking
- Raise the head of your bed
- Wear loose fitting clothing around your stomach
Uses
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain foods and beverages
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- kidney disease
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- children under 12 years: ask a doctor
Other information
-
TAMPER EVIDENT: Do Not Use If The Carton Or Printed Foil Under Cap Is Open or Torn
- store at 20° to 25°C (68° to 77°F)
- avoid excessive heat or humidity
- this product is sugar free
- USP Dissolution Test Pending.
Inactive ingredients
croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin
Questions?
call 1-855-274-4122
Read the directions and warnings before use. Keep the carton. It contains important information including tips for managing heartburn.
Distributed by:
AUROHEALTH LLC
2572 Brunswick Pike
Lawrenceville, NJ 08648
Made in India
Code: TS/DRUGS/22/2009
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg Container (24 Tablets Bottle)
AUROHEALTH
NDC 58602-734-07
MAXIMUM STRENGTH
Ranitidine Tablets USP,
150 mg
ACID REDUCER
PREVENTS & RELIEVES
HEARTBURN associated with
acid indigestion and sour stomach
24 TABLETS
(24 DOSES)

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg Container Carton (24's Tablets)
AUROHEALTH
NDC 58602-734-07
*Compare to the active
ingredient in Zantac 150®
MAXIMUM STRENGTH
Ranitidine
Tablets USP,
150 mg
ACID REDUCER
PREVENTS & RELIEVES
HEARTBURN associated with
acid indigestion and sour stomach
24 TABLETS
(24 DOSES)

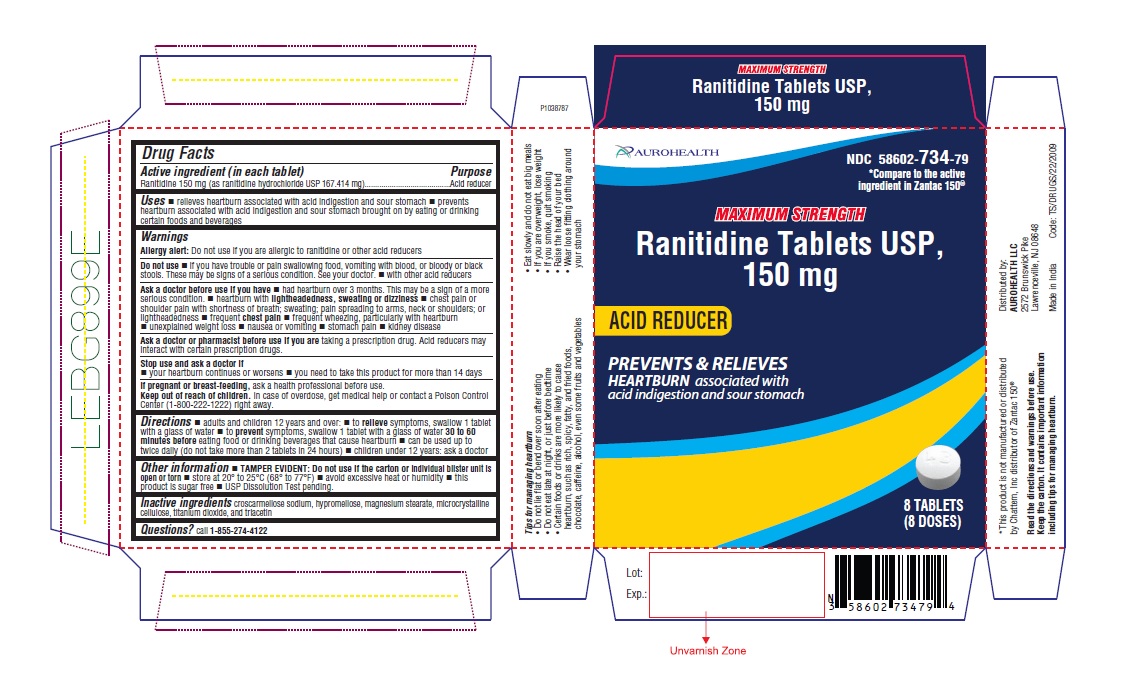
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg Blister Carton (8's (1 x 8) Tablets)
AUROHEALTH
NDC 58602-734-79
*Compare to the active
ingredient in Zantac 150®
MAXIMUM STRENGTH
Ranitidine Tablets USP,
150 mg
ACID REDUCER
PREVENTS & RELIEVES
HEARTBURN associated with
acid indigestion and sour stomach
8 TABLETS
(8 DOSES)
| MAXIMUM STRENGTH RANITIDINE
ranitidine tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Aurohealth LLC (078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurobindo Pharma Limited | 650381903 | ANALYSIS(58602-734) , MANUFACTURE(58602-734) | |