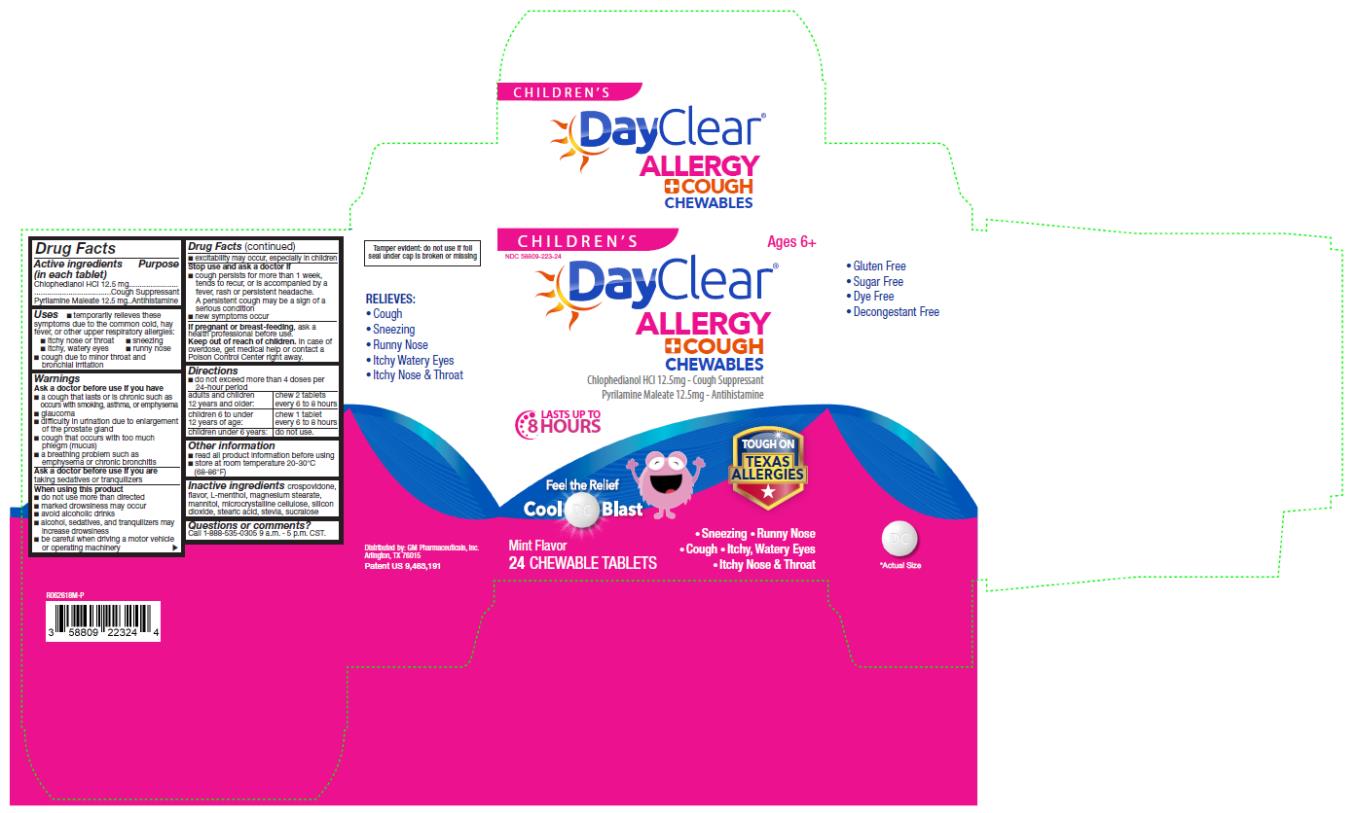
CHILDRENS DAYCLEAR ALLERGY COUGH CHEWABLES- chlophedianol hydrochloride, pyrilamine maleate tablet, chewable
GM Pharmaceuticals, INC
----------
Childrens DayClear Allergy Cough Chewables
Uses
■ temporarily relieves these symptoms due to the common cold, hay fever, or other upper respiratory allergies:
■ itchy nose or throat ■ sneezing
■ itchy, watery eyes ■ runny nose
■ cough due to minor throat and bronchial irritation
Warnings
Ask a doctor before use if you have
■ a cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
■ glaucoma
■ difficulty in urination due to enlargement of the prostate gland
■ cough that occurs with too much phlegm (mucus)
■ a breathing problem such as emphysema or chronic bronchitis
When using this product
■ do not use more than directed
■ marked drowsiness may occur
■ avoid alcoholic drinks
■ alcohol, sedatives, and tranquilizers may increase drowsiness
■ be careful when driving a motor vehicle or operating machinery
■ excitability may occur, especially in children
Directions
■ do not exceed more than 4 doses per 24-hour period
adults and children 12 years and older: chew 2 tablets every 6 to 8 hours
children 6 to under 12 years of age: chew 1 tablet every 6 to 8 hours
children under 6 years: do not use.
Other information
■ read all product information before using
■ store at room temperature 20-30°C (68-86°F)
| CHILDRENS DAYCLEAR ALLERGY COUGH CHEWABLES
chlophedianol hydrochloride, pyrilamine maleate tablet, chewable |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - GM Pharmaceuticals, INC (793000860) |