Label: CALCIUM ACETATE capsule
- NDC Code(s): 53746-590-01, 53746-590-05, 53746-590-20
- Packager: Amneal Pharmaceuticals of New York LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 31, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CALCIUM ACETATE CAPSULES safely and effectively. See full prescribing information for CALCIUM ACETATE CAPSULES.
CALCIUM ACETATE capsules, for oral use
Initial U.S. Approval: 1990INDICATIONS AND USAGE
- Calcium acetate capsules are a phosphate binder indicated for the reduction of serum phosphorus in patients with end stage renal disease. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Capsule: 667 mg calcium acetate capsule. (3)
CONTRAINDICATIONS
- Hypercalcemia. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
- The most common (> 10%) adverse reactions are hypercalcemia, nausea and vomiting. (6.1)
- In clinical studies, patients have occasionally experienced nausea during calcium acetate therapy. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypercalcemia
5.2 Concomitant Use with Medications
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Ciprofloxacin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
Patients with end stage renal disease may develop hypercalcemia when treated with calcium, including calcium acetate. Avoid the use of calcium supplements, including calcium-based nonprescription antacids, concurrently with calcium acetate.
An overdose of calcium acetate may lead to progressive hypercalcemia, which may require emergency measures. Therefore, early in the treatment phase during the dosage adjustment period, monitor serum calcium levels twice weekly. Should hypercalcemia develop, reduce the calcium acetate dosage, or discontinue the treatment, depending on the severity of hypercalcemia.
More severe hypercalcemia (Ca >12 mg/dL) is associated with confusion, delirium, stupor and coma. Severe hypercalcemia can be treated by acute hemodialysis and discontinuing calcium acetate therapy.
Mild hypercalcemia (10.5 to 11.9 mg/dL) may be asymptomatic or manifest as constipation, anorexia, nausea, and vomiting. Mild hypercalcemia is usually controlled by reducing the calcium acetate dose or temporarily discontinuing therapy. Decreasing or discontinuing Vitamin D therapy is recommended as well.
Chronic hypercalcemia may lead to vascular calcification and other soft-tissue calcification. Radiographic evaluation of suspected anatomical regions may be helpful in early detection of soft tissue calcification. The long term effect of calcium acetate on the progression of vascular or soft tissue calcification has not been determined.
Hypercalcemia (>11 mg/dL) was reported in 16% of patients in a 3-month study of solid dose formulation of calcium acetate; all cases resolved upon lowering the dose or discontinuing treatment.
Maintain the serum calcium-phosphorus (Ca x P) product below 55 mg2/dL2.
5.1 Hypercalcemia
Patients with end stage renal disease may develop hypercalcemia when treated with calcium, including calcium acetate. Avoid the use of calcium supplements, including calcium-based nonprescription antacids, concurrently with calcium acetate.
An overdose of calcium acetate may lead to progressive hypercalcemia, which may require emergency measures. Therefore, early in the treatment phase during the dosage adjustment period, monitor serum calcium levels twice weekly. Should hypercalcemia develop, reduce the calcium acetate dosage, or discontinue the treatment, depending on the severity of hypercalcemia.
More severe hypercalcemia (Ca >12 mg/dL) is associated with confusion, delirium, stupor and coma. Severe hypercalcemia can be treated by acute hemodialysis and discontinuing calcium acetate therapy.
Mild hypercalcemia (10.5 to 11.9 mg/dL) may be asymptomatic or manifest as constipation, anorexia, nausea, and vomiting. Mild hypercalcemia is usually controlled by reducing the calcium acetate dose or temporarily discontinuing therapy. Decreasing or discontinuing Vitamin D therapy is recommended as well.
Chronic hypercalcemia may lead to vascular calcification and other soft-tissue calcification. Radiographic evaluation of suspected anatomical regions may be helpful in early detection of soft tissue calcification. The long term effect of calcium acetate on the progression of vascular or soft tissue calcification has not been determined.
Hypercalcemia (>11 mg/dL) was reported in 16% of patients in a 3-month study of solid dose formulation of calcium acetate; all cases resolved upon lowering the dose or discontinuing treatment.
Maintain the serum calcium-phosphorus (Ca x P) product below 55 mg2/dL2.
-
6 ADVERSE REACTIONS
Hypercalcemia is discussed elsewhere [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical studies, calcium acetate has been generally well tolerated.
Calcium acetate was studied in a 3-month, open-label, non-randomized study of 98 enrolled ESRD hemodialysis patients and an alternate liquid formulation of calcium acetate was studied in a two week double-blind, placebo-controlled, cross-over study with 69 enrolled ESRD hemodialysis patients. Adverse reactions (>2% on treatment) from these trials are presented in Table 1.
Table 1: Adverse Reactions in Patients with End-Stage Renal Disease Undergoing Hemodialysis
Preferred Term
Total adverse reactions reported for calcium acetate
n=167
n (%)3-mo, open-label study of calcium acetate n=98
n (%)Double blind, placebo-controlled, cross-over study of liquid calcium acetate
n=69Calcium acetate
n (%)Placebo
n (%)Nausea
6 (3.6)
6 (6.1)
0 (0)
0 (0)
Vomiting
4 (2.4)
4 (4.1)
0 (0)
0 (0)
Hypercalcemia
21 (12.6)
16 (16.3)
5 (7.2)
0 (0)
Mild hypercalcemia may be asymptomatic or manifest itself as constipation, anorexia, nausea, and vomiting. More severe hypercalcemia is associated with confusion, delirium, stupor, and coma. Decreasing dialysate calcium concentration could reduce the incidence and severity of calcium acetate-induced hypercalcemia. Isolated cases of pruritus have been reported, which may represent allergic reactions.
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or to establish a causal relationship to drug exposure.
The following additional adverse reactions have been identified during post-approval of calcium acetate: dizziness, edema, and weakness.
-
7 DRUG INTERACTIONS
The drug interaction of calcium acetate is characterized by the potential of calcium to bind to drugs with anionic functions (e.g., carboxyl, and hydroxyl groups). Calcium acetate may decrease the bioavailability of tetracyclines or fluoroquinolones via this mechanism.
There are no empirical data on avoiding drug interactions between calcium acetate or calcium acetate capsules and most concomitant drugs. When administering an oral medication with calcium acetate capsules where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy, administer the drug one hour before or three hours after calcium acetate capsules or calcium acetate. Monitor blood levels of the concomitant drugs that have a narrow therapeutic range. Patients taking anti-arrhythmic medications for the control of arrhythmias and anti-seizure medications for the control of seizure disorders were excluded from the clinical trials with all forms of calcium acetate.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Calcium acetate capsules contain calcium acetate. Animal reproduction studies have not been conducted with calcium acetate, and there are no adequate and well controlled studies of calcium acetate use in pregnant women. Patients with end stage renal disease may develop hypercalcemia with calcium acetate treatment [see Warnings and Precautions (5.1)]. Maintenance of normal serum calcium levels is important for maternal and fetal well being. Hypercalcemia during pregnancy may increase the risk for maternal and neonatal complications such as stillbirth, preterm delivery, and neonatal hypocalcemia and hypoparathyroidism. Calcium acetate treatment, as recommended, is not expected to harm a fetus if maternal calcium levels are properly monitored during and following treatment.
8.3 Nursing Mothers
Calcium acetate capsules contain calcium acetate and is excreted in human milk. Human milk feeding by a mother receiving calcium acetate is not expected to harm an infant, provided maternal serum calcium levels are appropriately monitored.
8.5 Geriatric Use
Clinical studies of calcium acetate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Calcium acetate, USP acts as a phosphate binder. Its chemical name is calcium acetate, USP. Its molecular formula is C4H6CaO4, and its molecular weight is 158.17. Its structural formula is:
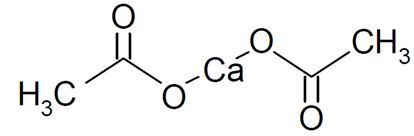
Each capsule has a light-blue cap imprinted with “AMNEAL” and white body imprinted with “590” with black ink. Each capsule contains 667 mg calcium acetate, USP (anhydrous; Ca(CH3COO)2; MW=158.17 grams) equal to 169 mg (8.45 mEq) calcium. Each capsule also contains the following inactive ingredients: FD&C Blue #1, FD&C Red #3, gelatin, magnesium stearate, polyethylene glycol and titanium dioxide. In addition to the inactive ingredients listed above, each capsule contains Opacode (Black) monogramming ink. Opacode (Black) contains D&C Yellow #10, FD&C Blue #2, FD&C Red #40, iron oxide black and shellac. Opacode (Black) may also contain ethanol, methanol, n-butyl alcohol and propylene glycol.
Calcium acetate capsules, USP are administered orally for the control of hyperphosphatemia in end-stage renal failure.
Meets USP dissolution test 4.
-
12 CLINICAL PHARMACOLOGY
Patients with ESRD retain phosphorus and can develop hyperphosphatemia. High serum phosphorus can precipitate serum calcium resulting in ectopic calcification. Hyperphosphatemia also plays a role in the development of secondary hyperparathyroidism in patients with ESRD.
12.1 Mechanism of Action
Calcium acetate, when taken with meals, combines with dietary phosphate to form an insoluble calcium phosphate complex, which is excreted in the feces, resulting in decreased serum phosphorus concentration.
12.2 Pharmacodynamics
Orally administered calcium acetate from pharmaceutical dosage forms is systemically absorbed up to approximately 40% under fasting conditions and up to approximately 30% under nonfasting conditions. This range represents data from both healthy subjects and renal dialysis patients under various conditions.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
Effectiveness of calcium acetate in decreasing serum phosphorus has been demonstrated in two studies of the calcium acetate solid oral dosage form.
Ninety-one patients with end-stage renal disease who were undergoing hemodialysis and were hyperphosphatemic (serum phosphorus >5.5 mg/dL) following a 1-week phosphate binder washout period contributed efficacy data to an open-label, non-randomized study.
The patients received calcium acetate 667 mg tablets at each meal for a period of 12 weeks. The initial starting dose was 2 tablets per meal for 3 meals a day, and the dose was adjusted as necessary to control serum phosphorus levels. The average final dose after 12 weeks of treatment was 3.4 tablets per meal. Although there was a decrease in serum phosphorus, in the absence of a control group the true magnitude of effect is uncertain.
The data presented in Table 2 demonstrate the efficacy of calcium acetate in the treatment of hyperphosphatemia in end-stage renal disease patients. The effects on serum calcium levels are also presented.
Table 2: Average Serum Phosphorous and Calcium Levels at Pre-Study, Interim, and Study Completion Time points
Parameter
Pre-Study
Week 4b
Week 8
Week 12
p-valuec
Phosphorus (mg/dL)a
7.4 ± 0.17
5.9 ± 0.16
5.6 ± 0.17
5.2 ± 0.17
≤0.01
Calcium (mg/dL)a
8.9 ± 0.09
9.5 ± 0.10
9.7 ± 0.10
9.7 ± 0.10
≤0.01
a Values expressed as mean ± SE.
b Ninety-one patients completed at least 6 weeks of the study.
c ANOVA of difference in values at pre-study and study completion.There was a 30% decrease in serum phosphorus levels during the 12 week study period (p<0.01). Two-thirds of the decline occurred in the first month of the study. Serum calcium increased 9% during the study mostly in the first month of the study.
Treatment with the phosphate binder was discontinued for patients from the open-label study, and those patients whose serum phosphorus exceeded 5.5 mg/dL were eligible for entry into a double-blind, placebo-controlled, cross-over study. Patients were randomized to receive calcium acetate or placebo, and each continued to receive the same number of tablets as had been individually established during the previous study. Following 2 weeks of treatment, patients switched to the alternative therapy for an additional 2 weeks.
The phosphate binding effect of calcium acetate is shown in the Table 3.
Table 3: Serum Phosphorous and Calcium Levels at Study Initiation and After Completion of Each Treatment Arm
Parameter
Pre-Study
Post-Treatment
p-valueb
Calcium Acetate
Placebo
Phosphorus (mg/dL)a
7.3 ± 0.18
5.9 ± 0.24
7.8 ± 0.22
<0.01
Calcium (mg/dL)a
8.9 ± 0.11
9.5 ± 0.13
8.8 ± 0.12
<0.01
a Values expressed as mean ± SEM
b ANOVA of calcium acetate vs. placebo after 2 weeks of treatment.Overall, 2 weeks of treatment with calcium acetate statistically significantly (p<0.01) decreased serum phosphorus by a mean of 19% and increased serum calcium by a statistically significant (p<0.01) but clinically unimportant mean of 7%.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Calcium acetate capsules USP, 667 mg are available as hard gelatin capsules with white opaque body imprinted with “590” and light-blue opaque cap imprinted with “AMNEAL” with black ink. Each capsule contains 667 mg calcium acetate, USP (anhydrous; Ca(CH3COO)2; MW=158.17 grams) equal to 169 mg (8.45 mEq) calcium.
They are supplied as follows:
Bottles of 100: NDC 53746-590-01
Bottles of 200: NDC 53746-590-20
Bottles of 500: NDC 53746-590-05
STORAGE: Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Inform patients to take calcium acetate capsules with meals, adhere to their prescribed diets, and avoid the use of calcium supplements including nonprescription antacids. Inform the patients about the symptoms of hypercalcemia [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Advise patients who are taking an oral medication where reduction in the bioavailability of that medication would have clinically significant effect on its safety or efficacy to take the drug one hour before or three hours after calcium acetate.
Manufactured by:
Amneal Pharmaceuticals Pvt. Ltd.
Oral Solid Dosage Unit
Ahmedabad 382213, INDIADistributed By:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 07-2020-00
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALCIUM ACETATE
calcium acetate capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53746-590 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM ACETATE (UNII: Y882YXF34X) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM ACETATE 667 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ALCOHOL (UNII: 3K9958V90M) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) METHYL ALCOHOL (UNII: Y4S76JWI15) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color white, blue Score no score Shape CAPSULE Size 22mm Flavor Imprint Code AMNEAL;590 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53746-590-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2014 2 NDC:53746-590-20 200 in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2014 3 NDC:53746-590-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201658 10/08/2014 Labeler - Amneal Pharmaceuticals of New York LLC (123797875)


