Label: MUCINEX FAST-MAX DAY TIME SEVERE CONGESTION AND COUGH - MUCINEX FAST-MAX NIGHT TIME COLD AND FLU MAXIMUM STRENGTH- acetaminophen, dextromethorphan hydrobromide, diphenhydramine hydrochloride, guaifenesin, and phenylephrine hydrochloride kit
- NDC Code(s): 63824-558-40
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- ACTIVE INGREDIENT
-
Uses
- temporarily relieves
(DAY TIME only):
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- nasal congestion due to a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive (DAY TIME only)
- temporarily relieves these common cold and flu symptoms
(NIGHT TIME only):
- cough
- minor aches and pains
- headache
- nasal congestion
- sore throat
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes due to hay fever
- temporarily reduces fever (NIGHT TIME only)
- controls cough to help you get to sleep
- temporarily relieves
(DAY TIME only):
-
Warnings
Liver warning (NIGHT TIME only)
This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks daily while using this product
Allergy alert (NIGHT TIME only)
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning (NIGHT TIME only)
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist. (NIGHT TIME only)
- with any other product containing diphenhydramine, even one used on the skin (NIGHT TIME only)
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease (NIGHT TIME only)
- heart disease
- diabetes
- high blood pressure
- thyroid disease
- trouble urinating due to an enlarged prostate gland
- glaucoma (NIGHT TIME only)
- a breathing problem such as emphysema or chronic bronchitis (NIGHT TIME only)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin (NIGHT TIME only)
- taking sedatives or tranquilizers (NIGHT TIME only)
When using this product
- do not use more than directed
- excitability may occur, especially in children (NIGHT TIME only)
- marked drowsiness may occur (NIGHT TIME only)
- alcohol, sedatives, and tranquilizers may increase drowsiness (NIGHT TIME only)
- avoid alcoholic drinks (NIGHT TIME only)
- be careful when driving a motor vehicle or operating machinery (NIGHT TIME only)
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- symptoms do not get better within 7 days or occur with fever (DAY TIME only)
- pain, nasal congestion, or cough gets worse or lasts more than 7 days (NIGHT TIME only)
- fever gets worse or lasts more than 3 days (NIGHT TIME only)
- redness or swelling is present (NIGHT TIME only)
- new symptoms occur (NIGHT TIME only)
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or headache that lasts. These could be signs of a serious condition.
-
Directions
- do not take more than directed (see Overdose warning)
- do not take more than 12 caplets in any 24-hour period
- adults and children 12 years of age and over: take 2 caplets every 4 hours
- children under 12 years of age: do not use
- Other information
- Inactive ingredients (Mucinex FAST-MAX DAY TIME SEVERE CONGESTION & COUGH)
-
Inactive ingredients (Mucinex FAST-MAX NIGHT TIME COLD & FLU)
croscarmellose sodium, crospovidone, FD&C blue no. 1 aluminum lake, FD&C blue no. 2 aluminum lake, ferric oxide yellow, methacrylic acid – ethyl acrylate copolymer (1:1) type A, mica, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, sodium bicarbonate, talc, titanium dioxide
- Questions?
- SPL UNCLASSIFIED SECTION
-
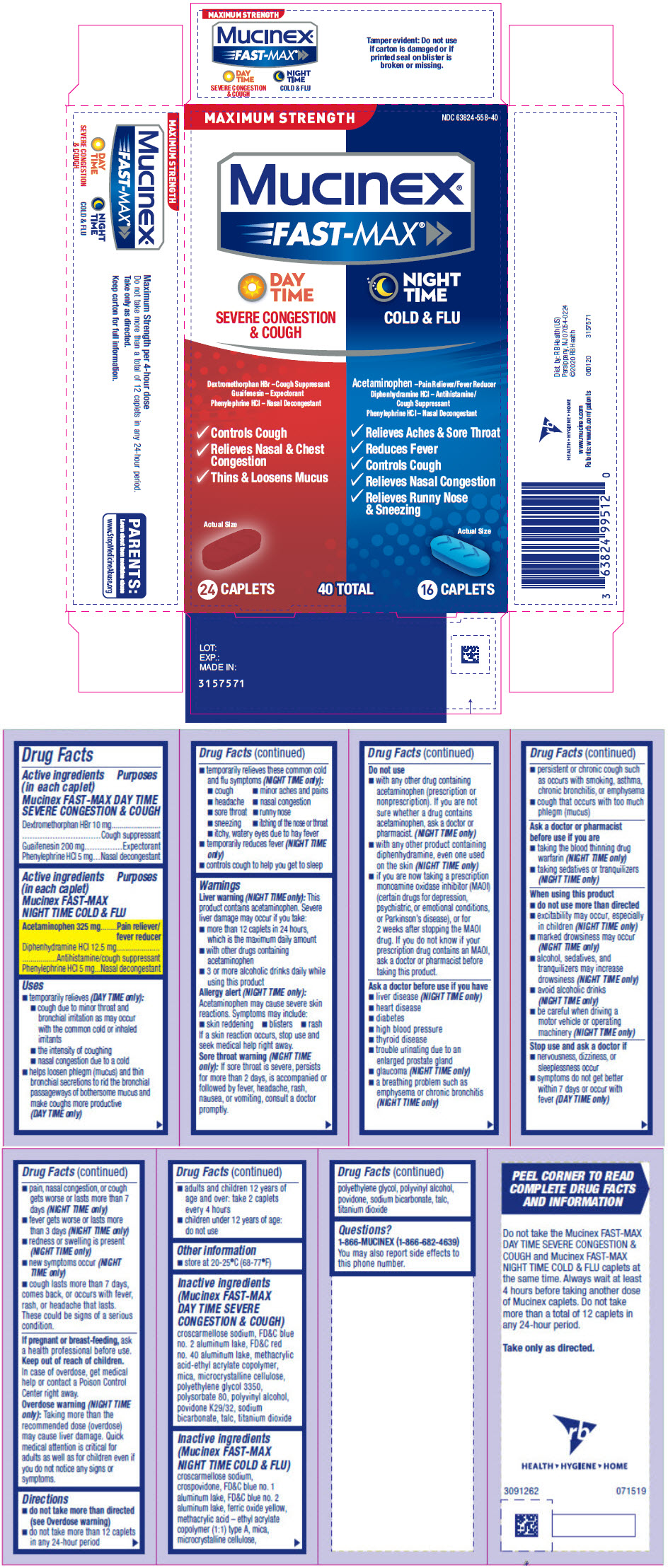
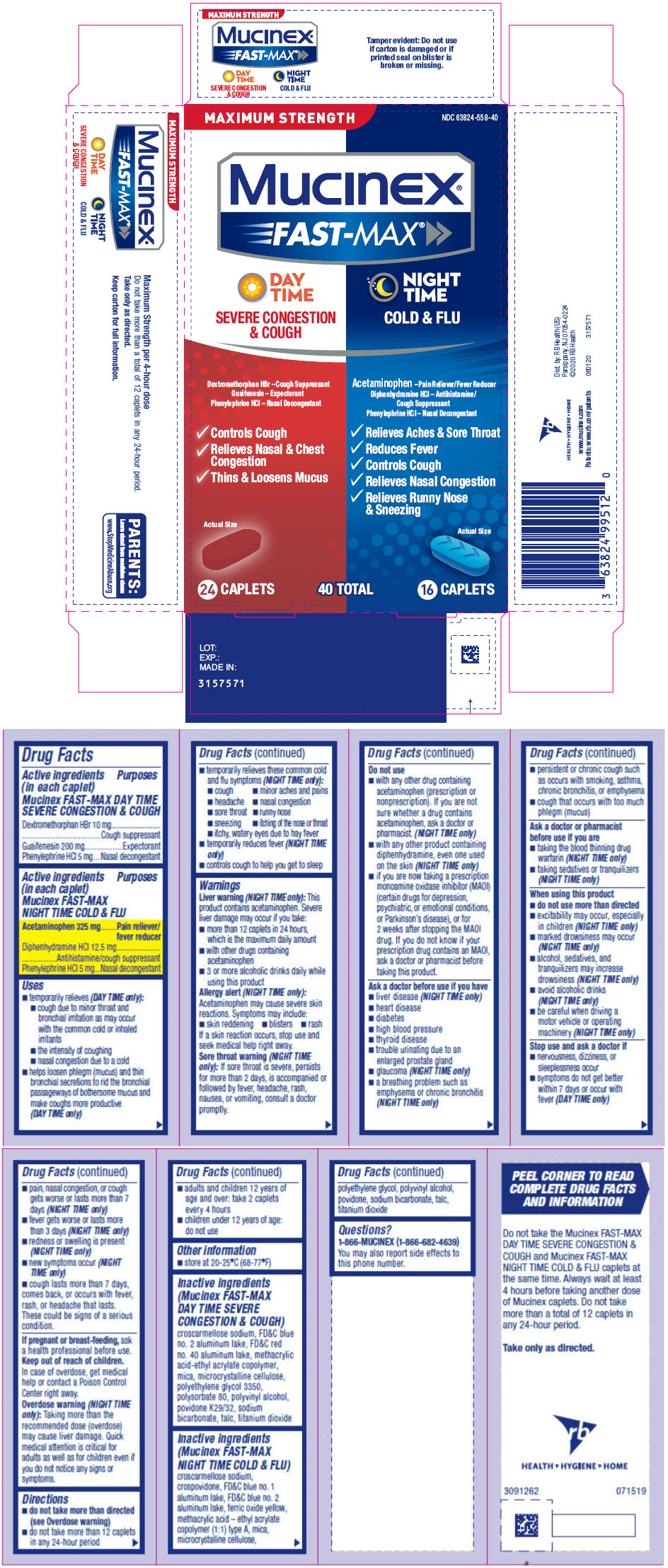
PRINCIPAL DISPLAY PANEL - Kit Carton
MAXIMUM STRENGTH
NDC 63824-558-40Mucinex®
FAST-MAX ®DAY
TIME
SEVERE CONGESTION
& COUGHDextromethorphan HBr – Cough Suppressant
Guaifenesin – Expectorant
Phenylephrine HCl – Nasal Decongestant- Controls Cough
- Relieves Nasal & Chest
Congestion - Thins & Loosens Mucus
Actual Size
NIGHT
TIME
COLD & FLUAcetaminophen – Pain Reliever/Fever Reducer
Diphenhydramine HCl – Antihistamine/
Cough Suppressant
Phenylephrine HCl – Nasal Decongestant- Relieves Aches & Sore Throat
- Reduces Fever
- Controls Cough
- Relieves Nasal Congestion
- Relieves Runny Nose
& Sneezing
Actual Size
24 CAPLETS
40 TOTAL
16 CAPLETS
-
INGREDIENTS AND APPEARANCE
MUCINEX FAST-MAX DAY TIME SEVERE CONGESTION AND COUGH - MUCINEX FAST-MAX NIGHT TIME COLD AND FLU MAXIMUM STRENGTH
acetaminophen, dextromethorphan hydrobromide, diphenhydramine hydrochloride, guaifenesin, and phenylephrine hydrochloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63824-558 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63824-558-40 4 in 1 CARTON 06/01/2020 1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 6 Part 2 1 BLISTER PACK 4 Part 1 of 2 MUCINEX FAST-MAX DAY TIME SEVERE CONGESTION AND COUGH MAXIMUM STRENGTH
dextromethorphan hydrobromide, guaifenesin, and phenylephrine hydrochloride tablet, coatedProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) ALUMINUM OXIDE (UNII: LMI26O6933) FD&C RED NO. 40 (UNII: WZB9127XOA) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE K30 (UNII: U725QWY32X) SODIUM BICARBONATE (UNII: 8MDF5V39QO) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape OVAL Size 20mm Flavor Imprint Code VVV;SCC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/01/2020 Part 2 of 2 MUCINEX FAST-MAX NIGHT TIME COLD AND FLU MAXIMUM STRENGTH
acetaminophen, diphenhydramine hydrochloride, and phenylephrine hydrochloride tablet, coatedProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) MICA (UNII: V8A1AW0880) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE K30 (UNII: U725QWY32X) SODIUM BICARBONATE (UNII: 8MDF5V39QO) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue Score no score Shape OVAL Size 20mm Flavor Imprint Code VVV;SI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/01/2020 12/14/2025 Labeler - RB Health (US) LLC (081049410)